Energy Balances with Phase Changes: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter.
Prepared by Mark D. Normand and Micha Peleg, Open content licensed under CC BY-NC-SA
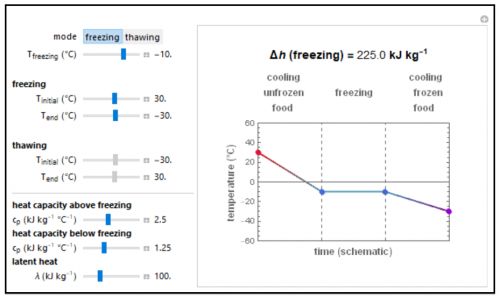
The net heat for freezing or thawing food is calculated using heat capacities and the heat of fusion. The initial, final, and freezing point values as well as the food’s thermal properties are entered with the sliders. The schematic graphical display below depicts the process on an arbitrary time scale in which the heating or cooling stages are shown as straight lines and the freezing or thawing stage as a horizontal line. Note that the temperature versus time relationships during heating or cooling are not actually linear. The Demonstration lets you enter combinations of thermal properties that might not be found in real foods.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- When a frozen food is warmed, what does a plot of temperature versus time look like approximately?
- Which requires more heat removal: a) cooling an unfrozen food from 25°C to 0°C, or b) freezing that food at 0°C. Assume the water is the major component of the food.