Isothermal CSTR: Interactive Simulation and optional Digital Experiment
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation. The optional digital experiment runs in your browser.
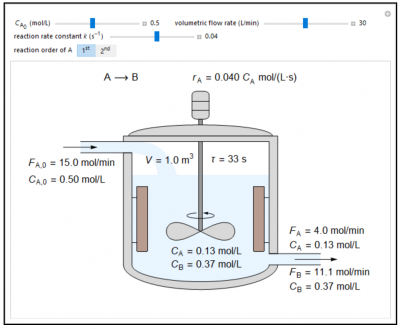
In this Demonstration, the liquid-phase reaction A → B takes place in an isothermal, continuous stirred-tank reactor (CSTR). Use the sliders to set the feed concentration of A, \(C_{A,0}\), the volumetric flow rate, and the rate constant k. Select the reaction order with respect to A using the “\(1^{st}\)” or “\(2^{nd}\)” button. The rate constant has the same numerical value when the reaction order changes, but its units are different. The figure shows the feed molar flow rate \(F_{A,0}\), the feed concentration CA,0, the outlet molar flow rates \(F_A, F_B\) and the outlet concentrations \(C_A, C_B\). Note that the outlet concentrations are identical to the concentrations in the reactor. The reactor residence time \(\tau = V/v\) is also calculated.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- How does the fraction of the feed that is converted to product in an isothermal CSTR change for a first-order reaction as the feed concentration increases?
- As the volumetric flow rate increases (and the residence time in the reactor decreases) for an isothermal CSTR, does the amount of product produced per time increase or decrease for a second-order reaction?
- When the concentration of product in an isothermal CSTR decreases, by changing the volumetric flow rate, does the rate of product formation (molar flow rate of B leaving the CSTR) increase or decrease for a first-order reaction?
Material Balances on a CSTR Digital Experiment