Multiple Steady States in a Non-Isothermal CSTR: ConcepTest and Example Problem
Try to answer this ConcepTest and solve the example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. We suggest that you write down the reasons for your answers. By the end of this module, you should be able to answer these on your own. Answers will be given at the end of this module.
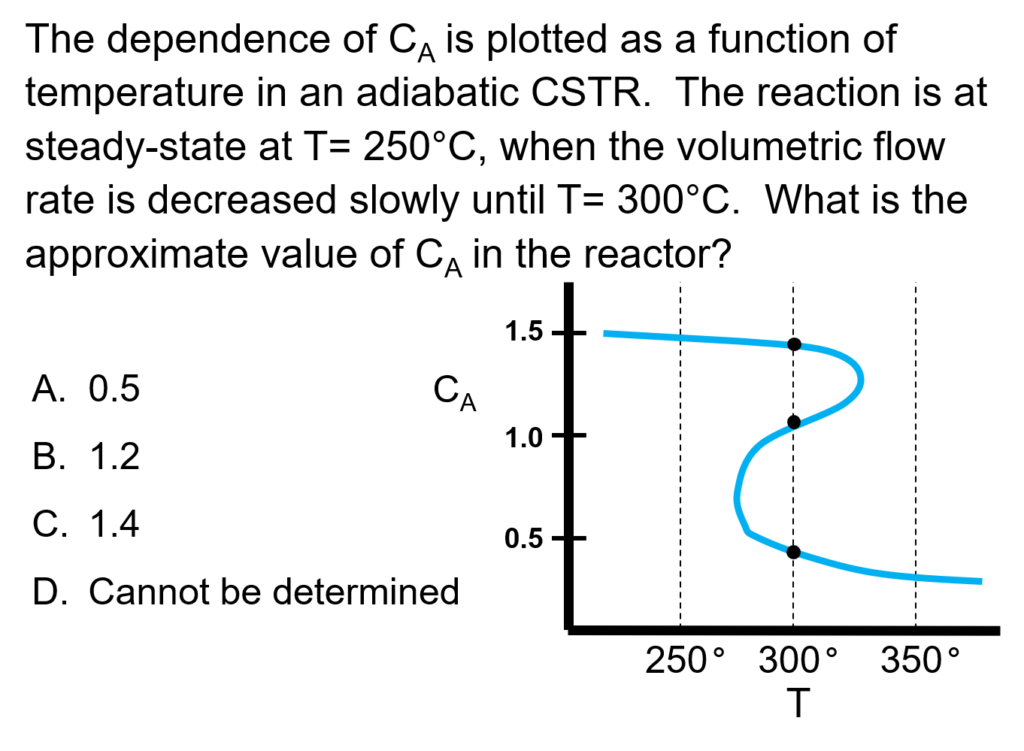
Example Problem 1
The reaction A + B → C takes place in a continuous stirred tank reactor (CSTR) that has a volume of 20 m3. The feed is at 295 K and contains propylene oxide (A), water (B), and methanol (M), each at a flow rate of 91 mol/h. The product (C) is propylene glycol. The volumetric flow rate to the reactor is 25 m3/h. The heat capacities in J/(mol K) are CPA = 146, CPB = 75, CPC = 192, and CPM = 79.
The reaction is first order in A, zero order in B; k = 6.9 x 1010e(-75,000/RT) h-1 where the activation energy is in units of J/mol. The heat of reaction is -56,800 J/mol.
The CSTR is surrounded by a cooling jacket and the heat transfer coefficient time the heat transfer area = 1.0 x 104 J/(h K). The cooling water temperature is 305 K and is assumed constant. What are the steady-state operating conditions for this CSTR?



