Properties of Fluids: Interactive Simulation
This simulation was prepared using Mathematica. Download the free CDF player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
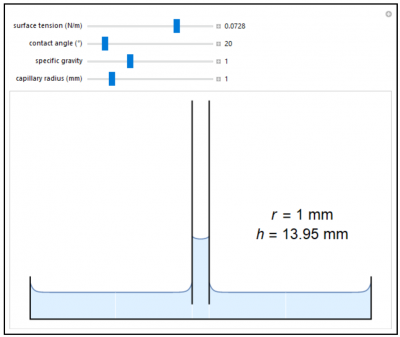
Liquids (such as water) that wet glass climb upward on the surfaces of their containers to form a concave meniscus. This occurs when adhesive solid-liquid intermolecular forces are stronger than liquid forces. Such liquids will rise in a narrow capillary tube until a balance is established between the effects of surface tension and gravity. The capillary rise increases sharply as the tube is made narrower.
This simulation was prepared by S. M. Blinder and is located here. Open content licensed under CC BY-NC-SA.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- As the contact angle increases from 10 degrees to 90 degrees, does the height of a column in a capillary increase or decrease?
- As the surface tension decreases, does the height of a column in a capillary change? How?
- Suppose we replace a fluid with one that is twice as dense but has the same surface tension and contact angle, does the height of a column in a capillary increase or decrease?
- Suppose a fluid in a tube with a diameter of 1 mm exhibits capillary action (height of fluid in tube is above the surrounding liquid level). Would the same fluid exhibit a height above the surrounding fluid if the tube diameter were 1 cm?