Recycle Mass Balances: ConcepTest and Example Problem
Try to answer this ConcepTest and solve the example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. We suggest that you write down the reasons for your answers. By the end of this module, you should be able to answer these on your own. Answers will be given at the end of this module.
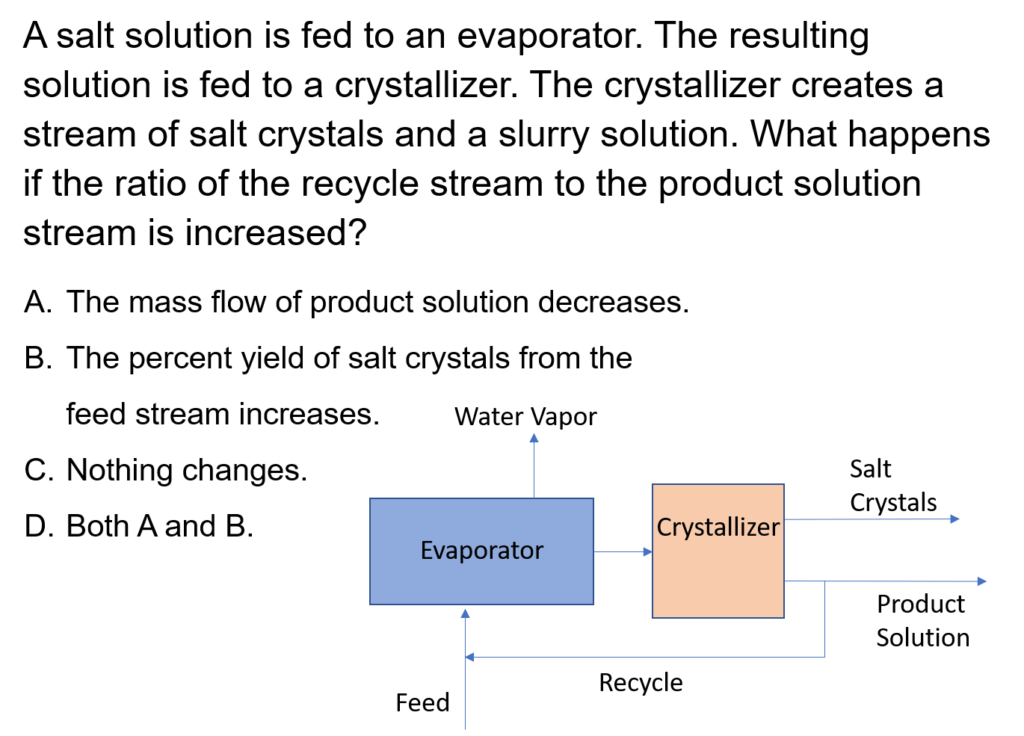
Example Problem 1
In an evaporative crystallization process, 400 kg/h of a 15 wt% KCl/85 wt% H2O feed is mixed with recycle and fed to an evaporator, which evaporators some of the water so the exit stream has a KCl mass fraction of 0.38. The resulting solution is fed into a crystallizer and filter. The resulting filter cake contains KCl crystals and saturated KCl solution, which is 28% KCl; the crystals are 84% of the total weight of the filter cake. The rest of the saturated KCl solution is recycled and mixed with the fresh feed. Calculate the flow rate of crystals leaving the system, the flow rate of water vapor, and the recycle flow rate.