Recycle Mass Balances: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
In an evaporative crystallization process, 400 kg/h of a 15 wt% KCl/85 wt% H2O feed is mixed with recycle and fed to an evaporator, which evaporators some of the water so the exit stream has a KCl mass fraction of 0.38. The resulting solution is fed into a crystallizer and filter. The resulting filter cake contains KCl crystals and saturated KCl solution, which is 28% KCl; the crystals are 84% of the total weight of the filter cake. The rest of the saturated KCl solution is recycled and mixed with the fresh feed. Calculate the flow rate of crystals leaving the system, the flow rate of water vapor, and the recycle flow rate.
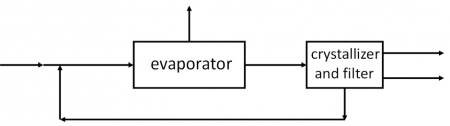
Example Problem 3
Potassium dichromate (K2Cr2O7) is to be recovered from 25 wt.% aqueous solution. The solution is joined by a recycle stream and fed to a crystallizer/centrifuge where enough water is removed so the solution is 85 wt.% water. Exiting the crystallizer are the crystals with 10% of the solution, and the remaining solution which forms the recycle stream. The filter cake, which contains 85 wt.% crystals and the res solution is fed to a dryer where it is contacted with dry air. The remaining water is evaporated, leaving pure potassium dichromate crystals. The air leaves the dryer with 0.08 mol fraction water. For a production rate of 1000 kg/h of potassium dichromate crystals, determine the: 1. water evaporated in the crystallizer/centrifuge 2. mass flow rate of the recycle stream and 3. moles of air that flow through the dryer.
Example Problem 2
The feed to a distillation column is a 45 mol % n-pentane – 55 mol % n-hexane liquid mixture. The vapor stream leaving the column, which contains 98 mol % pentane and the balance hexane, goes to a total condenser. Half of the liquid condensate is returned to the top of the column and the rest is withdrawn as product at a rate of 85 kmol/hr. The overhead product contains 95% of the pentane fed to the column.The liquid stream leaving the bottom of the column goes to a reboiler. Part of the stream is vaporized. The vapor is recycled to the bottom of the column and the liquid is taken off as a product as well. What is the molar flow rate of the feed stream and the molar flow rate and composition of the bottom liquid stream?
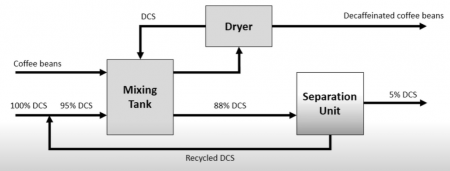
Example Problem 4
Each 100 kg of coffee beans contains 1.5 kg of caffeine. The solvent removes 90% of the caffeine in the coffee beans. For each 100 kg of coffee beans, 20 kg of DCS leaves with the coffee beans. 90% of the DCS is recovered through drying and is passed back into the mixing tank. The solvent entering the mixing tank is 95% DCS and that entering the settling unit is 88%. The waste solution leaving the settling unit contains 5% DCS. Find the amount of solvent entering per 100 kg coffee beans and the recycle stream compositions in weight fractions.