Ternary Phase Diagrams: Screencasts
A brief overview of using a triangular phase diagram for a tertiary system (acetone-water-MIBK).
We suggest you list the important points in this screencast as a way to increase retention.
Develops a ternary phase diagram using equilibrium data.
We suggest you list the important points in this screencast as a way to increase retention.
Optional screencast: Interpolating Tie Lines on a Ternary Diagram
Important Equations:
Gibbs Phase Rule for a non-reactive system: F = 2 + C – P
where F is the number of degrees of freedom, C is the number of components, and P is the number of phases.
Lever rule for two liquid phases (a , b) in equilibrium:
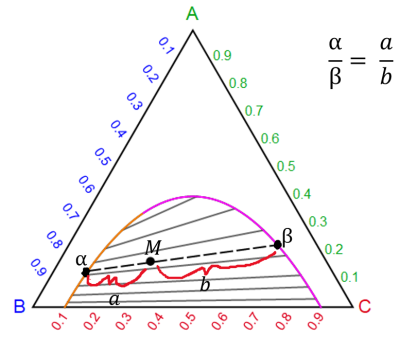
where a is the amount of the a phase, b is the amount of b phase, a and b are distances on the phase diagram, and M is the overall composition of the system.



