Chemical Potential: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
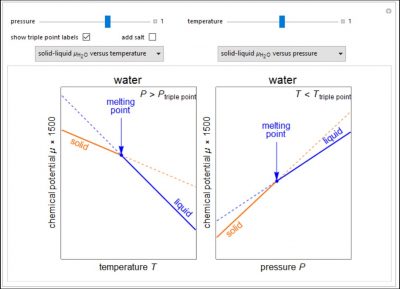
Changes in the chemical potential μ of water as a function of pressure P (at constant temperature T) or temperature (at constant pressure P) determine vapor-liquid, vapor-solid, and liquid-solid phase changes. Because the x-axes cover narrow ranges of temperature and pressure, the chemical potential plots are linear. Since the objective of this Demonstration is to show the qualitative behavior, the temperature or pressure is shown on dimensionless relative scales. Also, note that the y-axis scale is not the same for all plots so that the differences in chemical potential are easier to see. Chemical potential as a function of pressure is also shown for the solid-liquid phase change for ethanol, which has a different pressure dependence than water. Use the drop-down menus to select which two plots are displayed. The more stable phases (solid lines) have a lower chemical potential. Use sliders to set temperature for the μ versus P plots or to set pressure for the μ versus T plots. Note that the sliders change the temperature and pressure over narrow ranges. Check “add salt” to see the effect of adding salt to liquid water, and set the salt concentration with a slider. The phase changes that occur depend on the pressure or temperature relative to the triple point; check “show triple point labels” to show these on the plots.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- How is the behavior of solid water and solid ethanol different as pressure increases? Why?
- How does adding salt change the location of the melting point?
- If a gas is at a temperature below the triple point, what happens as the pressure increases at constant temperature?