Diffusion and Reaction in a Porous Catalyst: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download).
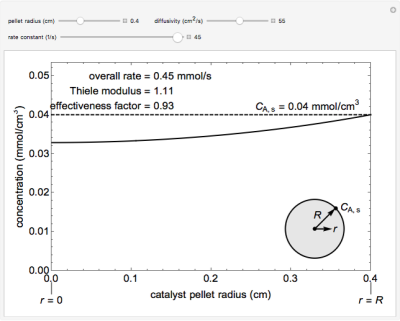
The overall rate of reaction in an isothermal, porous, spherical catalyst pellet is calculated for a first-order, gas-phase reaction that is limited by diffusion in the catalyst pores. This simulation plots the reactant concentration inside the catalyst pellet versus the pellet radius. Use the sliders to set the pellet radius, diffusivity and reaction rate constant. The Thiele modulus is a dimensionless number that represents the ratio of reaction rate to diffusion rate. The effectiveness factor is the overall rate of reaction divided by the rate of reaction if the entire catalyst were at CA,s = 0.04 mmol/cm3 (the external surface concentration). The diffusivity slider represents diffusivity DAB, not effective diffusivity De. The value used for pellet porosity \(\phi\) is 0.4, constriction factor \(\sigma\) is 0.8, and tortuosity \(\tau\) is 3.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- When the radius of a spherical catalyst increases, does the effectiveness factor increase, decrease, or not change? Why?
- To increase the effectiveness factor of a catalyst pellet, would you increase the rate constant or the diffusivity? Why?