Energy Balances with Reaction: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
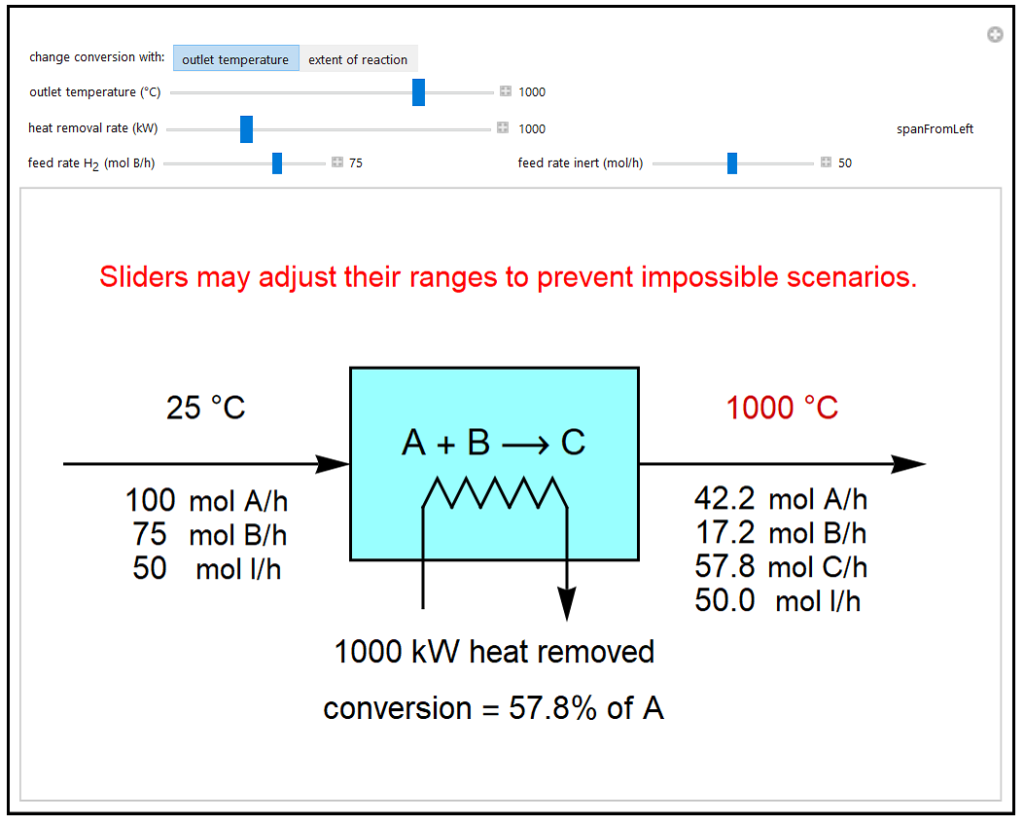
This simulation calculates material and energy balances for a reactor with heat transfer. The reaction A + B → C, is acetylene (A) hydrogenation to ethylene (C), where B is H2. The feed rate of A is fixed at 100 mol/h, and the feed rates of H2 (B) and an inert (I) are changed with sliders. Either the extent of reaction or the outlet temperature can be used to change the conversion of A. If the “extent of reaction” is chosen, then changing heat removal with the slider changes the outlet temperature. If “outlet temperature” is chosen, then the values of outlet temperature and heat removal selected determine the conversion. If the extent of reaction is restricted by the limiting reagent B, the process indicates when conditions are not possible. If the outlet temperature is below 0°C or above 1,000°C, the message “unrealistic operating conditions” appears.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- If the inert feed rate to the reactor increases while keeping the H2 feed rate, the outlet temperature, and the heat removal constant, what happens to the conversion?
- If the inert feed rate to the reactor increases while keeping the H2 feed rate, the conversion, and the heat removal rate constant, does the outlet temperature increase or decrease?