First Law - Open Systems: ConcepTest and Example Problem
Try to answer this ConcepTest and solve the example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. We suggest that you write down the reasons for your answers. By the end of this module, you should be able to answer these on your own. Answers will be given at the end of this module.
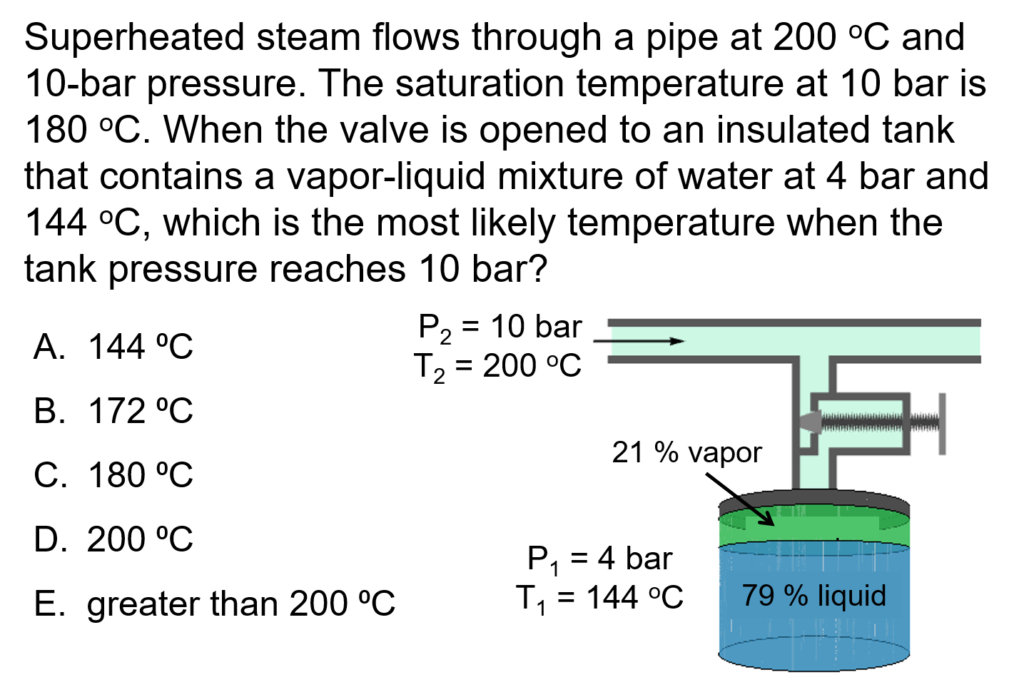
Example Problem 1
One kg of liquid water at 40°C is in a flask that is connected, through a valve, to a vacuum pump. Water evaporates when the valve is opened. The water vapor is removed fast enough that the flask can be considered adiabatic. As water evaporates, the remaining liquid water cools, and when it reaches 0°C, it starts to freeze. What fraction of the water will have evaporated when the rest is frozen?
