Mixing and Solution: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
One pound of a 20% H2SO4 solution at 60°F was mixed with a half-pound of an 80% H2SO4 solution at 60°F. How much heat must be removed to carry out this process isothermally?
Example Problem 3
2 lbm of pure water at 100°F is mixed adiabatically with 1 lbm of 60% H2SO4 solution at 100°F. Find the outlet temperature of the mixture.
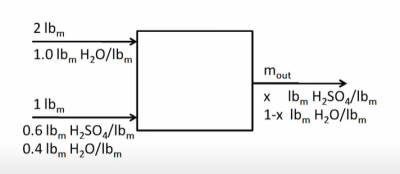
Example Problem 2
2 lbm of pure water at 100°F is mixed isothermally with 1 lbm of 60% H2SO4 solution at 100°F. Find the heat release upon mixing.

Example Problem 4
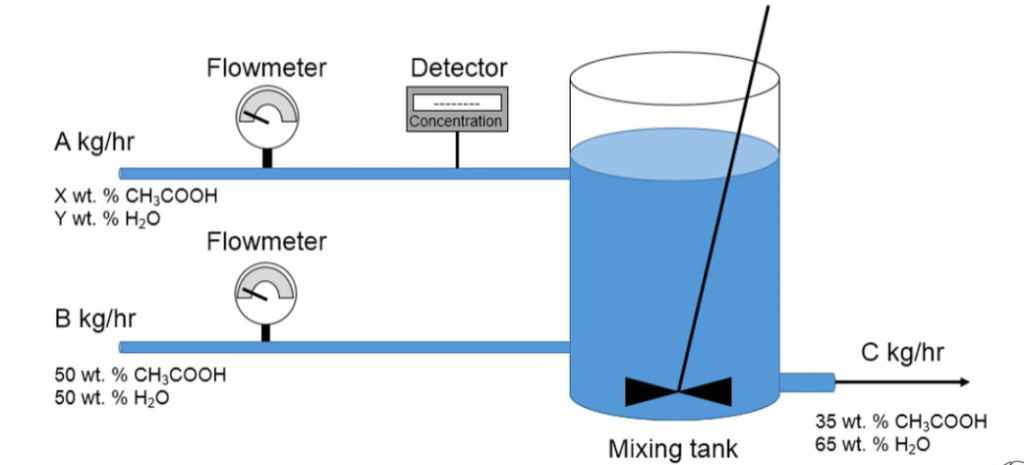
A mixing tank is used to prepare a solution for a reaction. The concentration and flow rate of stream A is variable, thus flow meters and a detector are used to ensure a specific concentration leaving the mixing tank. Use the schematic and information below to help an operator determine appropriate equations to use in their control operations for this process.