Multi-Stage Batch Distillation: Interactive Simulations
The first simulation will run on this page in your browser. A screencast that demonstrates how to use the simulation is below it. The second simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download).
Simulation: Multi-stage Batch Distillation
In this Demonstration, multi-stage batch distillation is simulated using animation. Ten kmol of a binary mixture (components A and B with B being the more volatile component) are placed in a batch still. First, use a slider to set the initial mole fraction of component B in the still. Next, adjust the number of trays in the column with the “equilibrium stages” slider, and adjust the reflux ratio of the column with the “reflux ratio (L/D)” slider. Specify the amount of liquid to be collected using the “amount to collect” slider. When you press “collect”, the liquid evaporates into a collection flask. After the specified amount of distillate is collected, the collection flask is set aside and an empty flask is substituted. This process can be repeated until 1.0 mol remains in the still or 8 flasks have been filled. The graphic on the right side may be changed by choosing an option next to “display”. Select “reset” to start over. Tooltips are available by hovering the mouse over stage trays in the column and over some lines in the plots.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For multi-stage batch distillation of a binary mixture without an azeotrope, what happens to the mole fraction of the distillate as the number of stages increases?
- For multi-stage batch distillation of a binary mixture without an azeotrope, what happens to the mole fraction of the distillate as the reflux ratio increases?
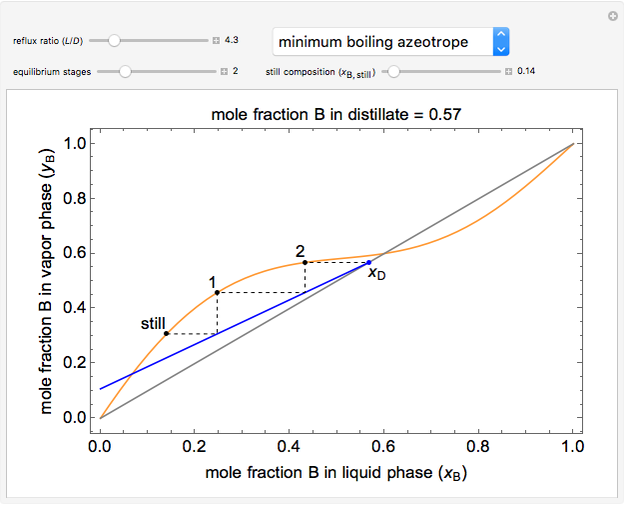
This simulation demonstrates how the reflux ratio, reboiler (still) composition, and number of equilibrium stages (all of which can be changed with sliders) affect the distillate composition (displayed above the graph) in a multi-stage batch-distillation (or continuous rectification) for a binary mixture. Each black dot on the y-x equilibrium curve denotes the composition at that equilibrium stage. Hover the mouse over a composition stage label (still, 1, 2, etc.) to display the composition at that stage. Select between a binary mixture with no azeotrope, a minimum-temperature azeotrope, or a maximum-temperature azeotrope. Stage 1 is defined as the first stage above the reboiler. This demonstration assumes constant molar overflow, 100% stage efficiency, and a complete condenser.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a minimum-boiling azeotrope at a mole fraction for component B of 0.60, as the number of stages increases, what happens to the mole fraction of the distillate?
- For a maximum-boiling azeotrope at a mole fraction for component B of 0.50, as the number of stages increases, what happens to the mole fraction of the distillate?