Purge Stream in a System with Recycle: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
N2 + 3H2 → 2NH3
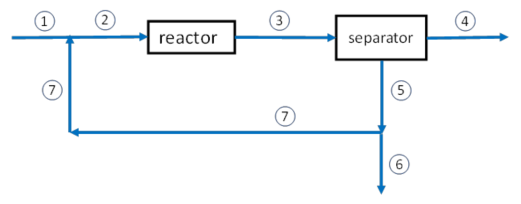
The reaction to produce ammonia (the Haber process) is shown above. A fresh feed containing stoichiometric amounts of the reactants as well as an inert gas combines with a recycle stream to form the feed to the reactor where a fractional conversion of nitrogen of 15% is attained. The products then enter a separator where the ammonia is removed and the unreacted gases as well as the inert are recycled back to the reactor. If the feed to the reactor is 10 mol/s of which 20% is the inert and the rest N2 and H2 in stoichiometric proportions, find the composition of the fresh feed.
Example Problem 2
A reactor system with a separator, recycle, and a purge is used is used to form NH3 from a feed stream that is 74.775 mol/s of H2, 24.925 mol/s of N2, and 0.30 mol/s of Ar. The reactor converts 40% of the N2 feed into NH3. The separator, located immediately after the reactor, removes all the NH3 but does not remove any H2, N2, or Ar. A purge stream then removes 1.00% of the H2, N2, Ar stream before it is recycled back to the reactor feed. How much NH3 is made per second?