Stripping Columns: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download).
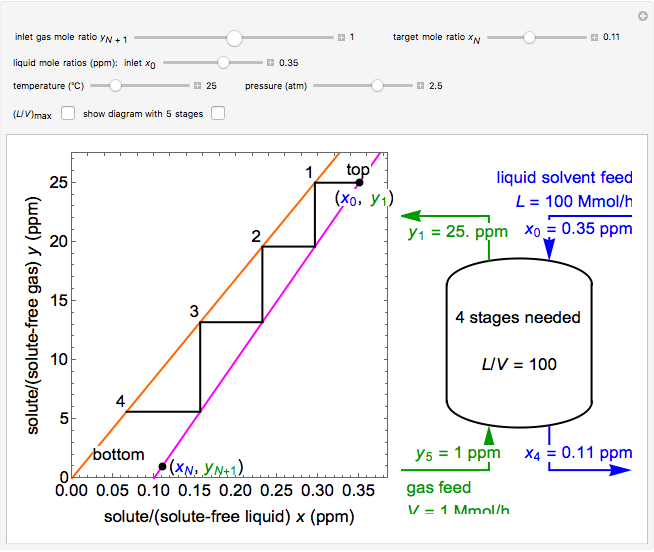
In this Demonstration, a trayed stripping column is used to remove an impurity from a liquid feed by stripping the impurity into a gas stream. The pink operating line is obtained from a mass balance. The phase equilibrium line, which is obtained from Henry’s law, is orange. The number of trays/stages needed to obtain a desired outlet solute mole ratio in the liquid stream is calculated.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- As the temperature increases, how does the slope of the equilibrium line change? How does the slope of the operating line change? How does the number of stage for a given separation change? Why?
- As the pressure increases, how does the slope of the equilibrium line change? How does the slope of the operating line change? How does the number of stage for a given separation change? Why?