Capillary Action

Description
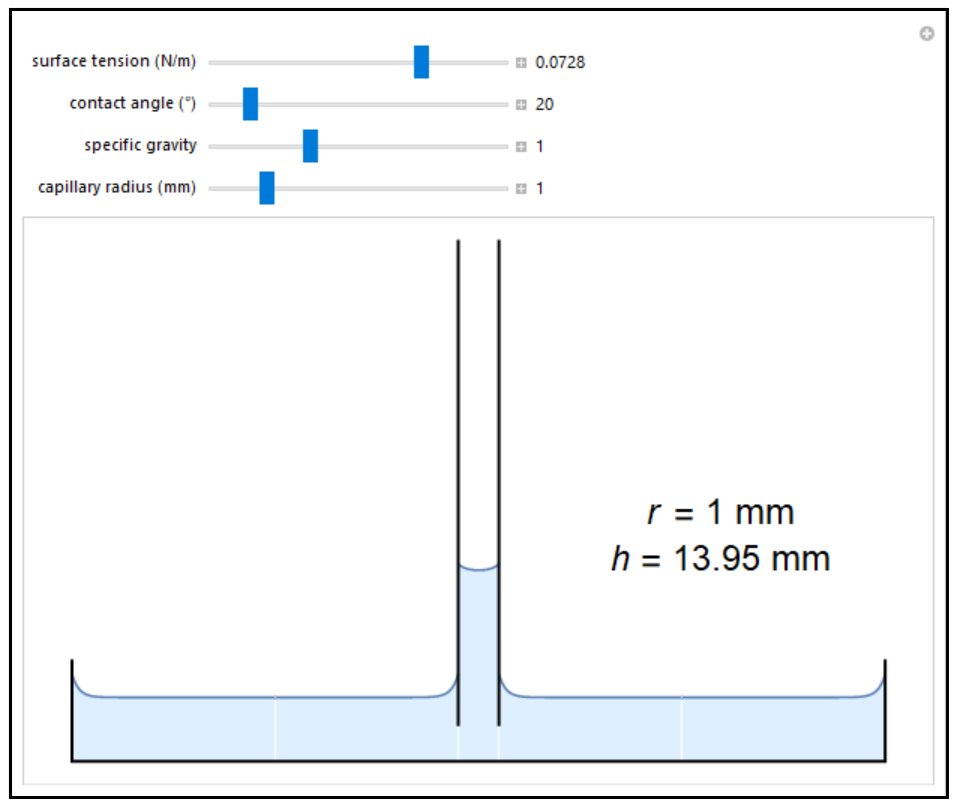
Liquids (such as water) that wet glass climb upward on the surfaces of their containers to form a concave meniscus. This occurs when adhesive solid-liquid intermolecular forces are stronger than liquid forces. Such liquids will rise in a narrow capillary tube until a balance is established between the effects of surface tension and gravity. The capillary rise increases sharply as the tube is made narrower.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About
Author: S. M. Blinder. Open content licensed under CC BY-NC-SA.
View the source code for this simulation



