Batch Reactors at Constant Volume or Constant Pressure

Description
Instructional video
Description
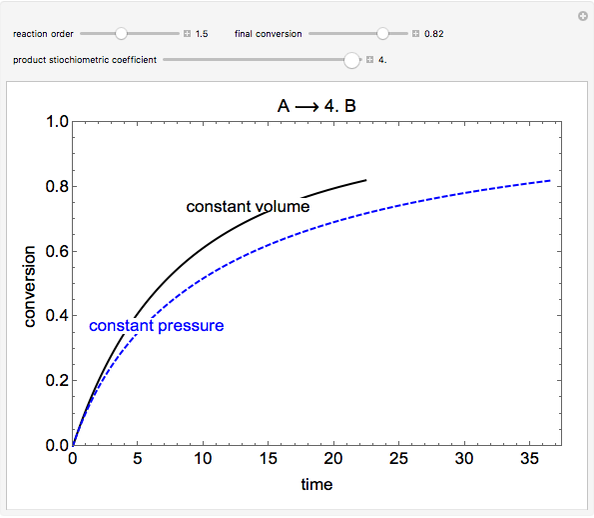
This simulation compares the time for an irreversible, gas-phase reaction to reach a certain conversion fraction in an isothermal batch reactor operating either at constant volume or at constant pressure. Both reactors start at the same initial conditions. If the reaction involves a mole change and the reaction is not zero or first-order, then the time to reach a certain conversion is different for the two types of reactors. In a constant-pressure reactor, the reactant concentration changes because of the volume change due to the mole change.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
View the source code for this simulation
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Rachael L. BaumannView the source code for this simulation
Instructional video



