Minimized Volume for Reactors in Series

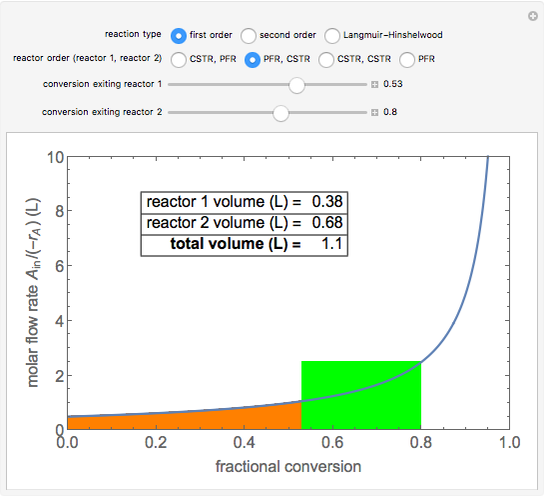
Two isothermal chemical reactors in series are used to carry out the reaction \( A \rightarrow B \). Each reactor can be a continuous stirred-tank reactor (CSTR) or a plug flow reactor (PFR). Choose which reactors are used and in which order. Then, select the fractional conversion from the second reactor by changing the conversion exiting the first reactor (which corresponds to changing the size of the first reactor). This determines the minimum total volume for this arrangement. The rate of disappearance of the reactant, \( -r_A \), depends on the reaction order (first or second order) or the rate expression (Langmuir–Hinshelwood, or L–H, mechanism). The L–H mechanism is a dual-site irreversible reaction \( A \cdot S + S \rightarrow B \cdot S + S \), where \( S \) corresponds to a surface site. The area on a plot of the ratio (molar feed flow rate/rate of reaction) versus fractional conversion (a Levenspiel plot) is proportional to the reactor volume. The PFR volume is proportional to the area under the curve (from inlet to outlet conversion), and the CSTR volume is the difference in conversion multiplied by the y-axis value at the reactor outlet.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Nicholas R. Larson
View the source code for this simulation



