Add a Component to a Mixture with an Azeotrope

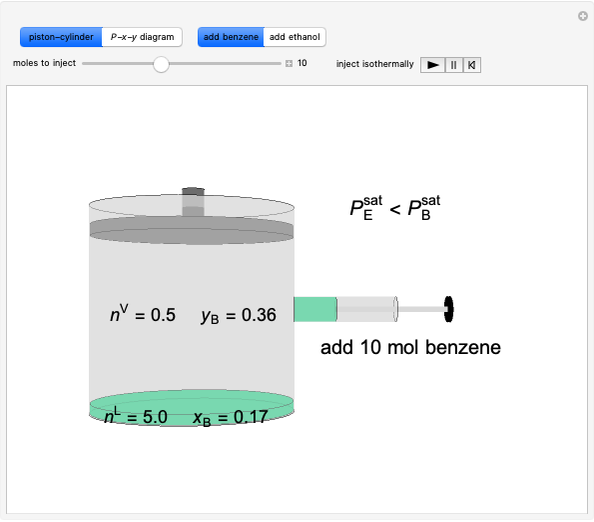
Benzene and ethanol, which have an azeotrope at a benzene mole fraction of xB=0.6, are in vapor-liquid equilibrium (VLE) inside a cylinder fitted with a piston. Add either benzene or ethanol to the system by clicking the play button next to “inject isothermally”. The final state when the system returns to equilibrium after injection depends on which component is injected and how much is injected. The piston-cylinder shows the relative amounts of liquid and vapor, and the total moles in the liquid phase, nL, and in the vapor phase, nV, are displayed. The mole fraction of benzene in the liquid, xB, and the vapor, yB are also displayed. The P-x-y diagram shows the results of the mass balances and demonstrates why a given phase is the final state. To observe behavior for injecting a different number of moles or to inject the other component, click reset before clicking “inject isothermally” again.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
