Adiabatic Evaporation of Water into Vacuum

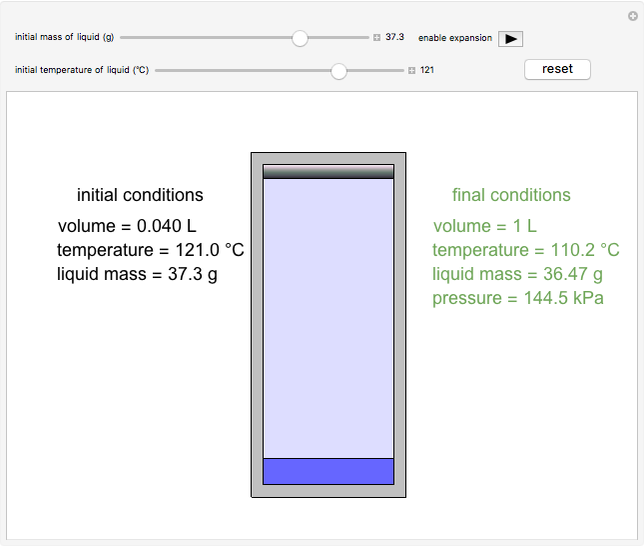
In this simulation, a cylinder fitted with a weightless, frictionless piston initially contains a liquid slightly above its saturation pressure; the volume above the piston is initially under vacuum. You can select the initial mass and temperature of the liquid using the sliders. The final volume is fixed at 1.0 L. Expansion is enabled when the orange stop is removed. The piston then moves to the top of the cylinder as some liquid evaporates, resulting in the final values of temperature, pressure and liquid mass, as shown. The temperature drops due to evaporative cooling, while the final pressure reaches its saturation value. The liquid volume in the cylinder is exaggerated relative to the vapor volume for better visualization.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Neil Hendren
View the source code for this simulation



