Circumnavigating the Critical Point

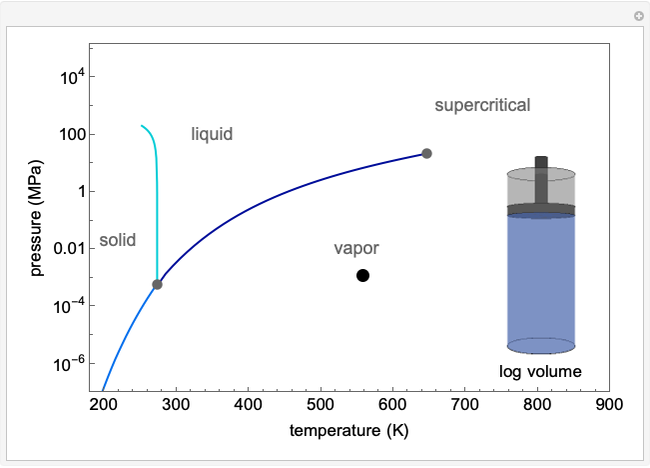
The pressure-temperature phase diagram for water is used to illustrate the concept of state functions and the possibility of going from the liquid phase to the vapor phase (or the other way) without a phase change (a single phase throughout the process) by circumnavigating the critical point, which is the highest temperature and pressure where two distinct phases exist (647 K, 22.1 MPa for water). The piston and cylinder represent the log of the volume, so that the large differences in volume between gas and liquid can be visualized. Fluid with higher density is shown darker. The liquid, vapor, and supercritical regions are labeled, but no definite boundaries exist between these regions, because the transitions are continuous when going around the critical point; however, a phase change is observed when crossing the phase boundary. You can drag the green dot on the plot to change pressure and temperature; the volume (obtained from the Peng-Robinson equation of state) is displayed. The dot can only be moved from 10-3 to 103 MPa and from the triple point to 720 K, so that the volume changes can be more easily displayed.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation



