High-Pressure Chemical Equilibrium

Description
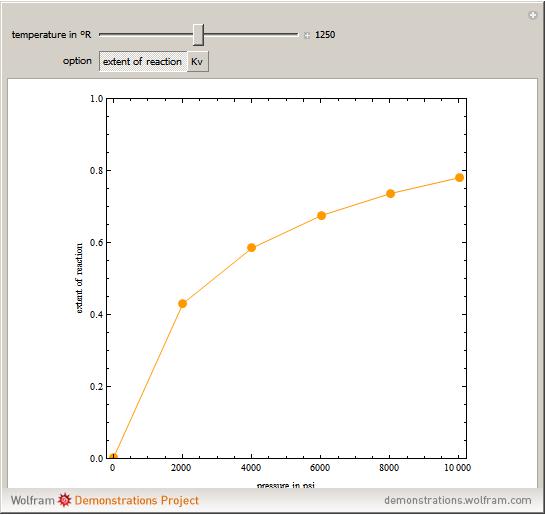
The Peng-Robinson equation of state is used to compute the correction factor due to gas-phase nonideality, Kv, as a result of the high pressure applied in the synthesis of ammonia (3H2 + N2 –> 2NH3). In addition, the extent of reaction is computed. The simulation plots these two quantities versus pressure for temperatures ranging from 1000°R to 1500°R. The extent of reaction is high and approaches one at high pressure and low temperature, as expected from LeChatelier’s rule. Kv is close to one at low pressure and is considerably smaller than one for high pressure, which indicates that this correction factor must be taken into account at high pressure.
About
Author: Housam Binous. Open content licensed under CC BY-NC-SA.
View the source code for this simulation



