Departure Functions: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
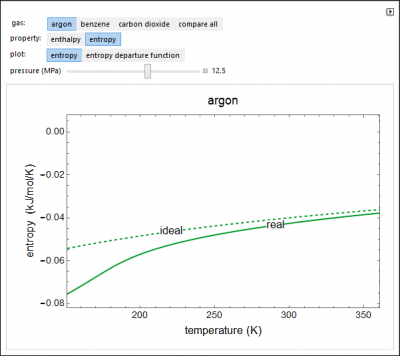
This demonstration plots the enthalpies and entropies for a real gas and an ideal gas as a function of temperature, relative to a reference state at a selected pressure and temperature, using the Peng-Robinson equation of state and ideal-gas heat capacities. Select argon, benzene, or carbon dioxide with buttons. For each gas, the temperature scale is adjusted so the plots are only shown above the critical temperature. Select enthalpy or entropy departure function, which is the difference between the thermodynamic property (enthalpy, entropy) for a real gas and an ideal gas at the same temperature and pressure. You can vary the pressure with the slider. Select “compare departure functions” to view departure functions for all three gases as a function of temperature at 10 MPa. Click “show labels” to label each departure function curve with the corresponding gas.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- How does the absolute value of the enthalpy departure function at a given temperature change as the pressure increases?
- At 600 K and 10 MPa, which gas would be expected to have the largest absolute value of its departure function: argon, carbon dioxide, or benzene?