Energy Balance in Steady-State PFR: Interactive Simulations
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Screencasts below explain how to use these simulations.
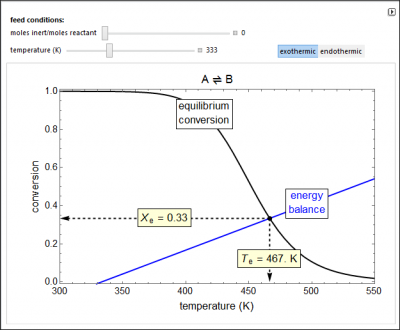
A reversible reaction, A ↔ B, takes place in an adiabatic plug-flow reactor (PFR). Select either an exothermic (ΔH < 0) or an endothermic (ΔH > 0) reaction. The black curve is the equilibrium conversion as a function of temperature from the van’t Hoff equation. The blue line is conversion as a function of temperature from the adiabatic energy balance. The intersection of the curve and the line is the conversion at equilibrium \((X_e)\) and the adiabatic temperature at equilibrium \((T_e)\). You can vary the feed temperature and the molar ratio of inert to reactant in the feed.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For an exothermic reaction in an adiabatic PFR, does equilibrium conversion increase or decrease when an inert is added to the feed? Does it matter if going to equilibrium?
- For an endothermic reaction in an adiabatic PFR, does equilibrium conversion increase or decrease when the feed temperature increases?
- For an exothermic reaction in an adiabatic PFR, does equilibrium conversion increase or decrease when the feed temperature increases?
This Demonstration plots the temperature and molar flow rate of the reactant as a function of distance down a plug flow reactor for an exothermic, gas-phase reaction. The reactor has heat exchange through the walls. Vary the feed temperature, activation energy for the reaction, and total molar flow rate with the sliders. Thermal runaway occurs at certain conditions and it is a sensitive function (parametric sensitivity) of the feed temperature and the activation energy.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- Suppose an exothermic reaction in a PFR with heat exchange has a maximum temperature 0.3 m down the reactor length. If the activation energy for the reaction were higher, would the maximum occur before or after 0.3 m?
- Suppose an exothermic reaction in a PFR with heat exchange has a maximum temperature of 700 K. If the activation energy for the reaction were higher, would the maximum temperature be higher, lower, or the same?
- Suppose an exothermic reaction in a PFR with heat exchange has a maximum temperature of 720 K at 0.5 m down the reactor. If the molar flow rate to the reactor increased, would the maximum temperature be higher, lower, or the same? Would the location of the maximum be before 0.5 m or after 0.5 m?