Fugacities of Mixtures: Interactive Simulations
These simulations were prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
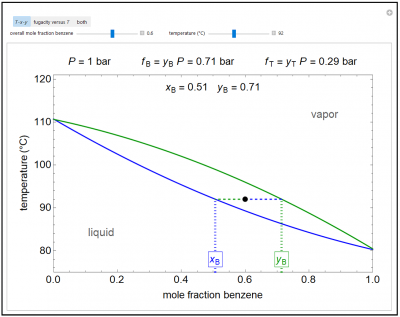
This Demonstration shows how the fugacities of benzene(B) and toluene(T) change with temperature and molar composition at constant pressure. Use the sliders to vary the temperature and overall mole fraction of benzene. Use the buttons to view the temperature-composition diagram (T-x-y), the fugacity-temperature plot, or both plots at once.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- Starting with an ideal liquid mixture, as temperature increases at constant pressure, how do the fugacities of each component change?
- When an ideal binary liquid mixture is heated at constant pressure to its bubble point and vapor starts to form, how do the fugacities of each component change as more vapor forms?
- Starting with an ideal gas mixture at constant pressure, how do the fugacities of each component change as the temperature increases?
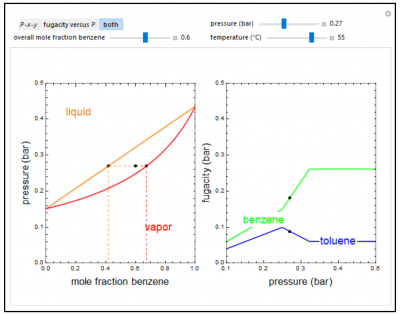
This Demonstration shows how the fugacities of benzene (B) and toluene (T) in a binary mixture change with pressure and composition at constant temperature. The liquid mixture is modeled as an ideal solution and the gas phase is assumed ideal, thus Raoult’s law models vapor-liquid equilibrium. Use sliders to vary the temperature and mole fraction of benzene. Use buttons to view the pressure-composition diagram (P-x-y), the fugacity-pressure plot or both plots at once. Black dots on the diagrams represent the pressure (on the P-x-y diagram) or fugacity (on the fugacity plot) for the given temperature and pressure.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a liquid mixture at 1 bar pressure, the fugacity of benzene is higher than the fugacity of toluene. Which fugacity is likely to be higher at higher pressure?
- As pressure increases for a vapor-liquid binary mixture (ideal solution) in equilibrium, do the fugacities of each component increase or decrease?
- As pressure increases for a binary vapor mixture, how do the fugacities of each component change?
The fugacities of water and carbon dioxide are calculated as a function of temperature for a closed container, which is a model of a can of soda. The concentrations of the two components are calculated in both the liquid and the gas phases. Change the temperature inside the can with a slider.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- As the temperature increases for the can of soda, what happens to the pressure?
- As the temperature increases for the can of soda, how do the fugacities of H2O and CO2 change?
- For a can of soda at 0°C, is the CO2 or the H2O fugacity higher in the liquid phase? How do you know this?