Heterogeneous Chemical Equilibrium: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
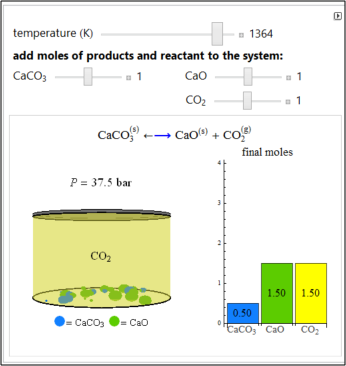
Solid calcium carbonate (CaCO3) decomposes into solid calcium oxide (CaO) and gaseous carbon dioxide (CO2) in a constant-volume container at high temperatures. Carbon dioxide is assumed to be an ideal gas, and the two solids are assumed to be in separate phases. You can vary the initial number of moles of CaCO3, CaO, and CO2 in the constant-volume container. The bar graph on the right shows the number of moles present at equilibrium. The equilibrium constant Keq changes as the temperature changes using the slider. The equilibrium constant is equal to the CO2 pressure (in bar) divided by the standard-state pressure of 1 bar.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For the conditions represented by the bar graph in the figure (0.5 mol CaCO3, 1.6 mol CaO, and 1.5 mol CO2,
(a) what happens when 0.5 mol CaO is added to the system at constant temperature? Does the pressure increase or decrease?
(b) what happens when 0.5 mol CaCO3 is added at constant temperature? Does the pressure increase or decrease?
(c) what happens when 0.5 mol CO2 is added at constant temperature? Does the pressure increase or decrease?
(d) what happens when 2 mol CO2 is added at constant temperature? Does the pressure increase or decrease? The reaction is endothermic. What happens to the pressure when the temperature increases?