Introduction to the First Law of Thermodynamics - Closed Systems: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
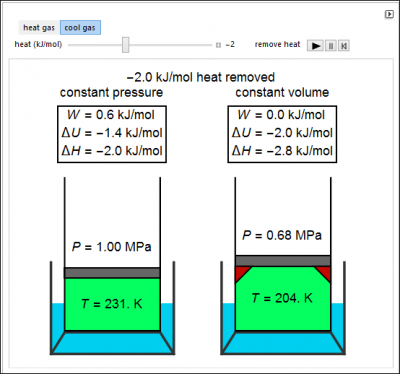
An ideal gas is heated or cooled in two containers, one at constant pressure and one at constant volume. The first law and the ideal gas law are used to calculate the final temperature, T, and the changes of internal energy and enthalpy for each system. The work is calculated for the constant-pressure process, and the final pressure is calculated for the constant-volume process.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- Which will produce more work when heated, a constant-pressure or constant-volume process?
- Which ends at a lower temperature when cooled, a constant-pressure or constant-volume process?