Isothermal Batch Reactors: Interactive Simulations
The first simulation will work on your browser. The second simulation was prepared using Mathematica. Download the free CDF player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
Simulation: Series Reactions in a Batch Reactor
Two first-order, liquid-phase reactions A → B → C take place in an isothermal batch reactor. The reactor initially contains only A at a concentration of 2 mol/L. The activation energy \(E_{a,2}\) of the second reaction (155 kJ/mol) is higher than the activation energy \(E_{a,1}\) of the first reaction (145 kJ/mol).
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a series reaction A → B → C in an isothermal batch reactor, how does the selectivity (moles of B)/(mole of C) change with time?
- For a series reaction A → B → C in an isothermal batch reactor, the activation energy for the first reaction is lower than the activation energy for the second reaction. How does the selectivity (moles of B)/(mole of C) change as the temperature increases?
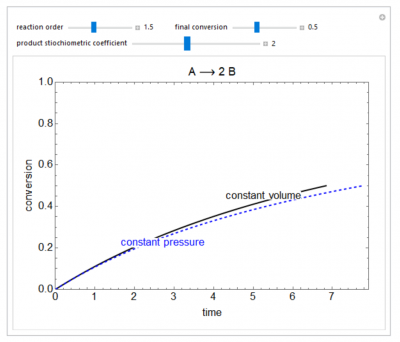
This Demonstration compares the time for an irreversible, gas-phase reaction to reach a certain fractional conversion in an isothermal batch reactor operating either at constant volume or at constant pressure. Both reactors start at the same initial conditions. If the reaction involves a mole change and the rate of reaction is not zero or first-order, then the time to reach a certain conversion is different for the two types of reactors; in a constant-pressure reactor, the reactant concentration changes because of the volume change due to the mole change.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a first-order, gas-phase reaction (A → 2B) starting at the same conditions in a constant-pressure and a constant-volume reactor, which reactor reaches 50% conversion first? Both reactors are initially the same size.
- For a second-order, gas-phase reaction (A → 2B) starting at the same conditions in a constant-pressure and a constant-volume reactor, which reactor reaches 50% conversion first? Both reactors are initially the same size.
- For a second-order, gas-phase reaction (A → B) starting at the same conditions in a constant-pressure and a constant-volume reactor, which reactor reaches 50% conversion first? Both reactors are initially the same size.