Isothermal Plug Flow Reactors (PFRs): Interactive Simulations
The first simulation works in your browser. The second and third simulations were prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
Simulation: Isothermal Plug Flow Reactor
This Demonstration illustrates the liquid-phase reaction A + 2B → C taking place in an isothermal plug flow reactor. Only A and B enter the reactor. Use the “inlet mole fraction A” slider to set the mole fraction of A in the feed. The reaction is first order in the concentration of A; you can vary the reaction order with respect to B and the rate constant k. When you vary the reaction order, the units of the rate constant change but its numerical value is constant. The molar flow rates of A, B and C \((F_A, F_B, F_C)\) are plotted versus the cumulative reactor volume.
Try to answer this question before determining the answer with the simulation. We suggest that you write down the reason for your answer.
- For the reaction A + 2B → C, suppose the feed contains only A and B and the ratio of A to B is 4. What is the main component in the reactor outlet?
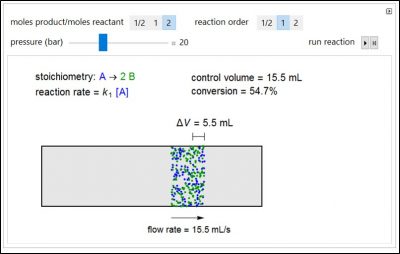
This Demonstration shows how the reaction stoichiometry affects the volumetric flow rate in an isothermal, constant-pressure, plug flow reactor by following a control volume (initially 10 mL of A) through the reactor. In the gas phase reaction A → nB, the reactant A is blue and the product B is green. Use the buttons to change the reaction stoichiometry n and reaction order. Use the slider to change the reactor pressure. The rate constants are chosen so that the initial rates are the same for all orders at 10-bar pressure. The inlet velocity is fixed at 1.4 cm/s, independent of pressure, so the feed molar flow rates change when pressure changes. Click the “Play” button to run the reaction and click the “Reset” button to start over. The volumetric flow rate is shown below the reactor, and the change in the 10-mL inlet volume is shown above the reactor.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a first-order, gas-phase reaction A → 2B, how does the volumetric flow rate change with distance down the PFR?
- Consider two first-order, gas-phase reactions in two identical isothermal PFRs. The initial rates are the same for each reaction. Which reaction has the higher conversion? A → 2B, A → 1/2 B
The irreversible reaction A → B takes place in an isothermal plug flow reactor (PFR). The rate of reaction and the fractional conversion are plotted versus space velocity (entering volumetric flow rate/reactor volume). As space velocity increases, the time available for reaction decreases. Use sliders to vary the dimensionless “rate constant” and dimensionless “feed concentration”. Select the “reaction order” with buttons. Conversion is low for smaller space velocities, regardless of the order of reaction, and for a first-order reaction, conversion is independent of feed concentration.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- For a negative first-order reaction, how does conversion change as the space velocity increases at very low space velocities for an isothermal PFR?
- For a zero-order reaction, how does the rate of reaction change as space velocity increases for an isothermal PFR?