Langmuir-Hinshelwood Kinetics: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
The irreversible, gas-phase reaction 2A → B + C is conducted over a solid catalyst. The reaction may be considered isothermal. The following reaction mechanism is proposed, where S represents a surface site.
- A + S ↔ A-S
- A-S + A-S → B-S + C + S
- B-S ↔ B + S
It is proposed that step 2 is rate-determining and irreversible, whereas steps 1 and 3 are equilibrated.
a. Based on the proposed mechanism, derive the Langmuir-Hinshelwood rate expression for the reaction.
b. Experiments with a differential reactor have been used to measure the rate of reaction. The results are shown below. Are the data consistent with the proposed mechanism? Under the experimental conditions, do you expect that the surface mainly consists of vacant sites, sites with adsorbed A, sites with adsorbed B, or sites with adsorbed C? Justify your answers.
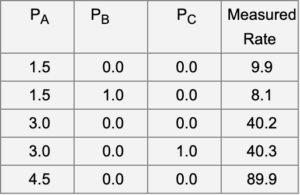
Note: Pressures are in MPa, measured rate is in arbitrary units.
Example Problem 3 (optional)
The Fe-catalyzed ammonia synthesis reaction (N2 +3H2 → 2NH3) is assumed to follow the Langmuir-Hinshelwood mechanism given below, where the first step is assumed rate-determining. Is such a mechanism consistent with an observation that the reaction is half-order in hydrogen pressure? Briefly explain. (* represents a surface site.)
N2 + 2* → 2N* (rate-determining)
H2 + 2* ↔ 2H*
N* + H* → NH* + *
NH* + H* → NH2* + *
NH2* + H* → NH3 + 2*
Example Problem 2
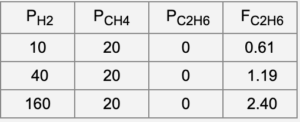
The gas-phase reaction C2H4 + H2 → C2H6 is conducted over a Ni catalyst. The mechanism for the reaction is proposed to be the following where * represents a surface site:
- C2H4 + * ↔ C2H4*
- H2 + 2* ↔ 2H*
- C2H4* + 2H* ↔ C2H6 + 3*
Reaction 3 is proposed to be rate-limiting (but reversible), with steps 1 and 2 being equilibrated.
a. Determine the Lanmuir-Hinshelwood expression for the rate of formation of ethane in terms of the partial pressures of reactants and product, the equilibrium constants for the three reactions K1, K2, and K3, the total concentration of surface sites, CT, and the forward rate constant for the surface reaction, k3.
b. The molar rate of ethane production is measured in a differential flow reactor for a series of inlet pressures (in Torr) as shown below. It is known that the hydrogen coverage on the surface is very low under these conditions. If this is the case, are the data consistent with the model? Explain.