Langmuir-Hinshelwood Kinetics: Interactive Simulation
This simulation was prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. A screencast below explains how to use this simulation.
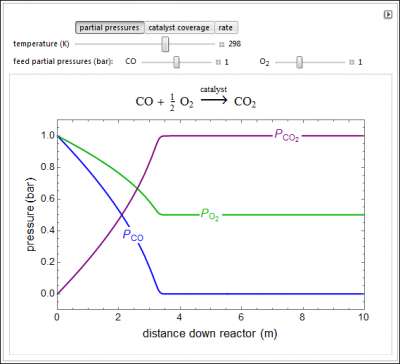
Oxygen oxidizes CO to CO2 on a supported metal catalyst in an isothermal plug flow reactor. Use buttons to select plots of “partial pressures” (P), “surface coverages” (θ), or “reaction rate” as a function of distance down the reactor. Use sliders to vary the “temperature”, the “CO feed partial pressure”, and the “O2 feed partial pressure”. The rate-determining step is the reaction of adsorbed CO and adsorbed O, and CO adsorbs more strongly than O2.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
For the reaction CO + ½O2 → CO2:
- If the reaction takes place in a PFR and the feed is 50% CO and 50% O2, what is the surface covered with at long distance down the reactor?
- If the reaction takes place in a PFR and the feed is 67% CO and 33% O2, what is the surface covered with at long distance down the reactor?
- As the gas phase concentration of O2 increases for the catalytic oxidation of CO to CO2, what happens to the rate? Note that CO adsorbs more strongly than O2.
- As the gas phase concentration of CO increases for the catalytic oxidation of CO to CO2, what happens to the rate? Note that CO adsorbs more strongly than O2.