Multiple Steady States in a Non-Isothermal CSTR: Interactive Simulations
These simulations were prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
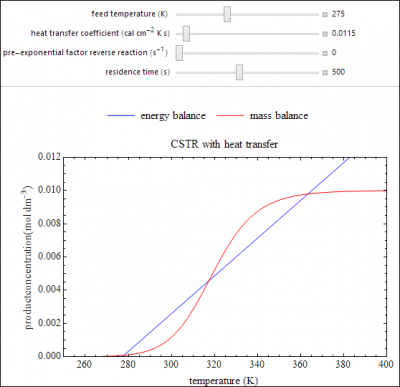
A continuously stirred tank reactor (CSTR) with heat transfer is used to carry out the reversible, exothermic reaction: A ⟷ B . This Demonstration shows how the steady-state solutions change as you vary the feed temperature, the heat transfer coefficient, and the pre-exponential factor for the reverse reaction. Plots of concentration of product B versus reactor temperature are shown for the mass balance and the energy balance; the intersections of these two balances correspond to the steady-state solutions for the reactor. Either one or three solutions are possible, and when three solutions are obtained, the middle solution (at the intersection of the two plots) is unstable.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- If the heat transfer coefficient increases for a steady-state, non-isothermal CSTR, does the likelihood of multiple steady states increase or decrease?
- For a reversible reaction in a non-isothermal CSTR operating at the higher-conversion steady state, as the feed temperature increases, what happens to the conversion?
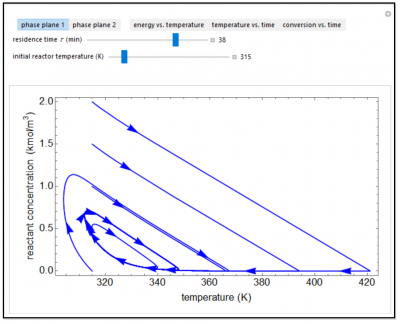
Under certain conditions, an exothermic reaction in a continuous stirred-tank reactor (CSTR) with heat exchange can exhibit multiple steady-state solutions. Some solutions are stable, others are unstable, and some can exhibit sustained oscillations or limit cycles. The steady-state operating conditions for the reactor depend on how the reactor is started up (initial conditions). The first phase plane plot shows reactant concentration versus reactor temperature as a function of time as the reactor approaches a steady state (or a limit cycle) for five initial concentrations; you can change the initial temperature, To, with a slider. The second phase plane plots conversion versus temperature for one set of initial conditions, and you can vary both the initial temperature and the initial concentration Co. Temperature and conversion are also shown as a function of time in separate plots for these initial conditions. The steady-state solutions change as you vary the residence time t, and limit cycle behavior is observed for residence times greater than about 30 minutes. The last plot (energy versus temperature) shows heat generated and heat removed as a function of temperature, and the intersections of these two curves correspond to steady-state solutions to the mass and energy balances for the CSTR.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- Does a non-isothermal CSTR always approach a steady state?
- If the steady-state operating temperature for a non-isothermal CSTR is 340 K and the initial reactor temperature is 300 K, can the reactor temperature be higher than 340 K as it approaches steady state?
- As the residence time increases for a non-isothermal CSTR, does the steady-state reactor temperature increase or decrease? conversion?