Recycle Mass Balances: Interactive Simulations
These simulations were prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
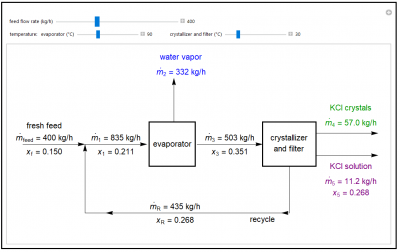
This demonstration shows mass balances for an evaporative crystallization process. A 15% potassium chloride/85% water feed is mixed with a recycle stream and fed to an evaporator, which evaporates water and increases the KCl concentration in the exiting solution. This solution enters a crystallizer and filter. A filter cake of KCl crystals and a portion of the saturated KCl solution are removed from the crystallizer and filter. The rest of the KCl solution is recycled and mixed with the fresh feed. Use sliders to set the fraction of crystals in the product stream, and the temperatures of the evaporator and crystallizer/filter. The solubility of KCl increases linearly with temperature.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- What happens when you increase the feed flow rate?
- How does increasing the temperature of the evaporator affect the amount of KCl solution leaving the crystallizer?
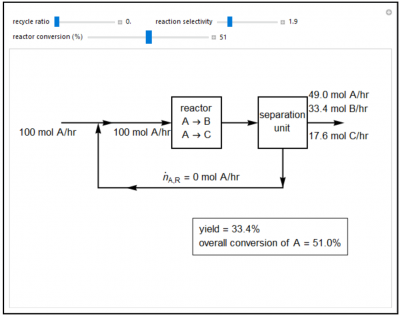
Mass balances are demonstrated for a reactor system. Pure A is fed to the system and two parallel reactions take place in the reactor:
A → B (desired) A → C (undesired)
A separation unit after the reactor removes some of the unreacted A and recycles it back to the feed stream. Use a slider to set the recycle ratio, which is the ratio of the recycle flow rate to the fresh feed flow rate. Set the reactor selectivity, which is the (flow rate of B) / (flow rate of C) leaving the reactor, with a slider. Set the reactor conversion, which is the percent of A entering the reactor that is consumed, with a slider. The overall conversion of A is the percent of the feed (100 mol A/hr) that is consumed. The yield is the percentage of the feed that is converted to the desired product B. A negative flow rate of A exiting the system indicates an impossible combination of reaction selectivity and reactor conversion.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- Is the overall conversion of A in a recycle reactor greater than or less than the reactor conversion?
- As the recycle ratio increases, what happens to the overall conversion?