Reversible and Irreversible Expansion/Compression Processes: ConcepTest and Example Problem
Try to answer this ConcepTest and solve this example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. By the end of this module, you should be able to answer these on your own. Answers will be given later in this module.
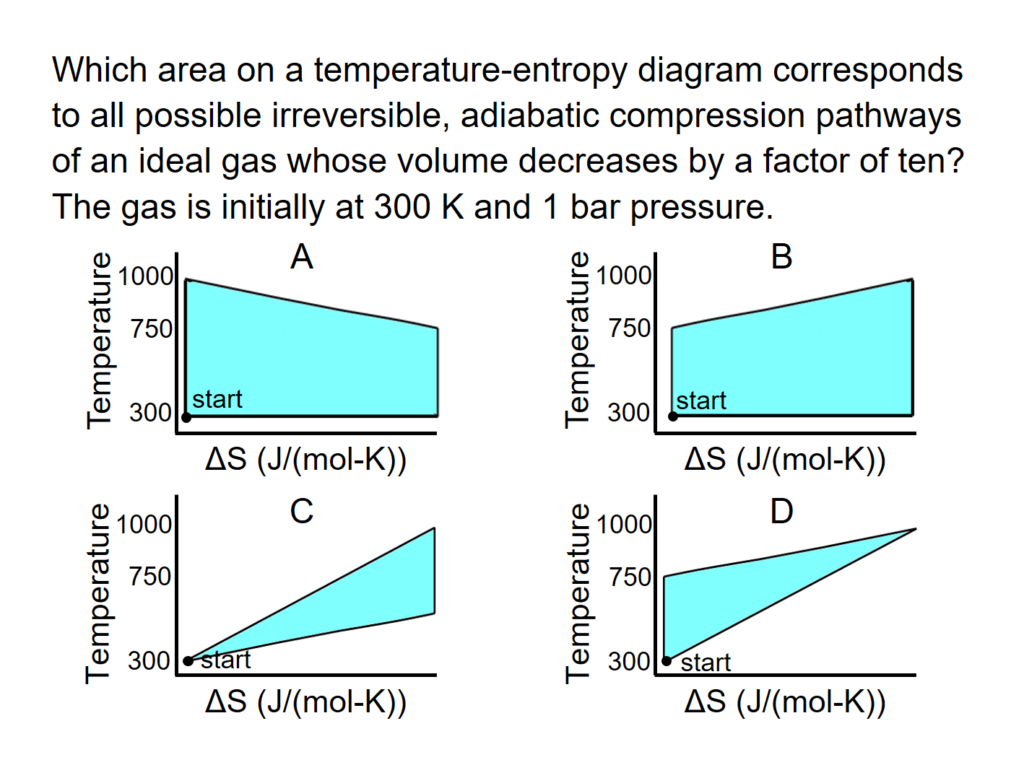
Example Problem 1
An ideal gas expands from 10 bar and 250°C to 1.5 bar and 150°C. The expansion is irreversible and adiabatic. What is the change in enthalpy for 10 mol of gas?
CP = 30 J/(mol K)
