Thermal Effects in Chemical Reactions: ConcepTest and Example Problem
Try to answer this ConcepTest and solve the example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. We suggest that you write down the reasons for your answers. By the end of this module, you should be able to answer these on your own. Answers will be given at the end of this module.
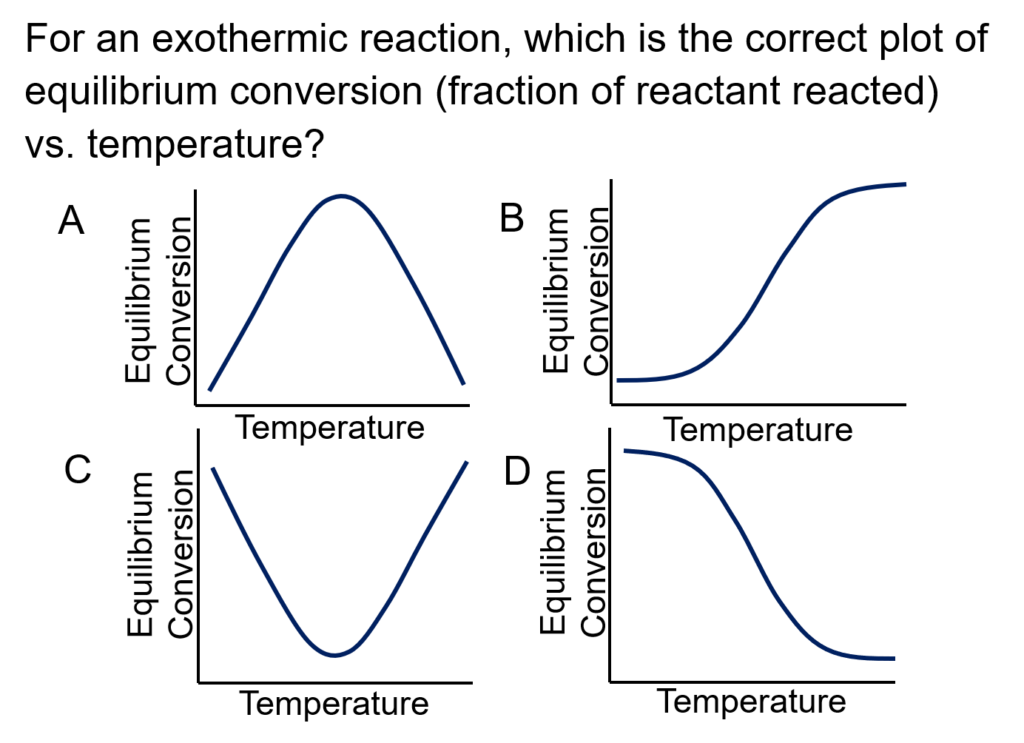
Example Problem 1
For the reversible, liquid phase reaction, A↔B, determine the adiabatic equilibrium temperature and the conversion when pure A is fed to the reactor at 300 K.
CPA = CPB = 50 cal /mol-K. Heat capacities are independent of temperature.
The equilibrium constant is 1.0 x 105 at 298 K; the heat of the reaction is -2.0 x 104 cal/mol.
