Vapor-Liquid Equilibrium for Non-Ideal Solutions: ConcepTest and Example Problem
Try to answer this ConcepTest and solve this example problem before using this module. Studies show that trying to answer the questions before studying material improves learning and retention. We suggest that you write down the reasons for your answers. By the end of this module, you should be able to answer these on your own. Answers will be given at the end of this module.
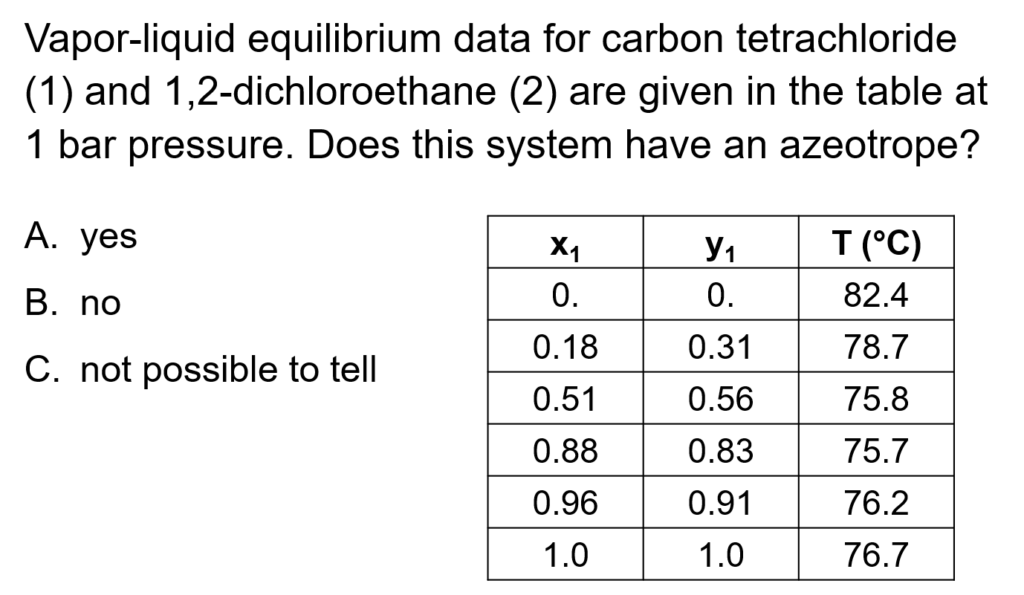
Example Problem 1
A vapor-phase mixture of components 1 and 2 is compressed at fixed temperature until the vapor completely condenses. The vapor contains 30 mol% component 1 and 70 mol% component 2. The saturation pressures for components 1 and 2 at the temperature of the system are 0.82 and 1.93 bar, respectively. The bubble pressure of a 50:50 mixture of components 1 and 2 is 1.08 bar.
a) Estimate the pressure require to completely liquify the 30:70 mixture, assuming that the excess Gibbs free energy of the liquid is well-modeled by the one-parameter Margules equation.
b) Would the required pressure to liquify the mixture be higher or lower if components 1 and 2 formed an ideal solution?
