Liquefaction: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
For N2 gas fed at 300 K and 1.0-bar pressure to a Linde liquefaction cycle, calculate the fraction of the flow entering the Joule-Thomson expansion at 200 bar and 165 K that is converted to liquid. The values in the table below were obtained from the Peng Robinson equation of state.
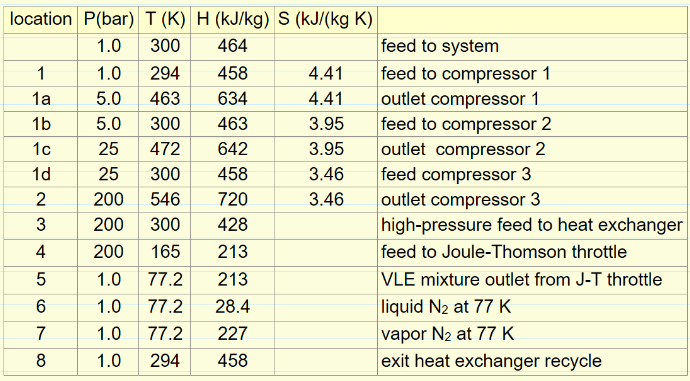
Example Problem 2
For N2 gas fed at 300 K and 1.0-bar pressure to a Linde liquefaction cycle, the gas enters the J-T expansion at 200 bar and 165 K. How much compressor work is required per kg of liquid N2 formed? The feed gas is compressed in 3 stages with cooling to 300 K after each stage. The outlet pressures from the three compressors are 5.0 bar, 25 bar, and 200 bar. The values in the table below were obtained from the Peng-Robinson equation of state.




