Purge Stream in a System with Recycle: Screencast
Explains what a purge stream is and why it is needed for a reactor system with recycle.
We suggest that after watching this screencast, you list the important points as a way to increase retention.
Important Equations:
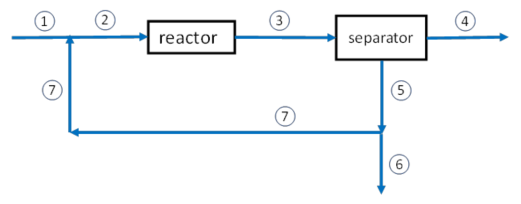
Material balances on mixing point
\(\dot{n}_2 = \dot{n}_1 + \dot{n}_7\) (Note that this equation is not independent of the balances of the individual components below.)
\(x_{i,2}\,\dot{n}_2 = x_{i,1}\,\dot{n}_1 + x_{i,7}\,\dot{n}_7\) (one equation for each component \(i\))
where \(\dot{n}_j\) = total molar flow rate at location \(j\) (\(j\) = 1 – 7)
\(x_{i,j}\) = mole fraction of component \(i\) at location \(j\)
Material balances on reactor
For reactants only and assuming a stoichiometric feed
\(x_{i,3}\,\dot{n}_3 = x_{1,2}\,\dot{n}_2 \cdot (1\, – \, X)\) (where component 1 is a reactant; one equation for each reactant)
For products only
\(x_{i,3}\,\dot{n}_3 = x_{1,2}\,\dot{n}_2 \cdot X \cdot a\) (where component 1 is a reactant; one equation for each product)
where \(a\) is the stoichiometric coefficient of the product
\(X\) = fractional conversion of reactants (stoichiometric feed)
For unreactive species
\(x_{i,2}\,\dot{n}_2 = x_{i,3}\,\dot{n}_3\)
Material balances on separator
(In general, a separator may not remove all of the reaction product and/or it may also remove some of the feed species.)
\(\dot{n}_3 = \dot{n}_4 + \dot{n}_5\) (Note that this equation is not independent of the balances of the individual components below.)
\(x_{i,3}\,\dot{n}_3 = x_{i,4}\,\dot{n}_4 + x_{i,5}\,\dot{n}_5\) (one equation for each component \(i\))
Material balances on splitting point
\(\dot{n}_5 = \dot{n}_6 + \dot{n}_7\) (Note that this equation is not independent of the balances of the individual components below.)
\(\dot{n}_6 = purge \cdot \dot{n}_5\)
\(\dot{n}_7 = (1\, -\, purge) \cdot \dot{n}_5\)
\(x_{i,5} = x_{i,6} = x_{i,7}\) (one equation for each component \(i\))
where \(purge\) = the fraction of stream 5 that is purged
Overall atom balances
(Note that these equations are not independent of the above equations.)
molar flow rate of atomic species in (stream 1) = molar flow rate of atomic species out (stream 4 + stream 6) for each atomic species in the feed



