Simple Batch Distillation of an Ethanol-Water Mixture

Description
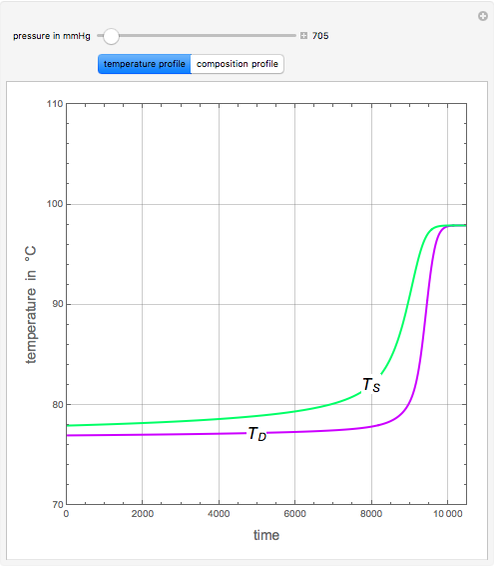
An equimolar binary mixture of ethanol and water is to be separated in a still pot that initially contains 0.575 kmol of the mixture. You can set the operating pressure, P, of this batch distillation, where P can vary from 700 to 900 mmHg so that the ideal gas assumption holds. This simulation plots the temperature of the liquid in the still, Ts (green), and the temperature of the distillate (i.e., condensed vapor exiting from the still), TD (magenta), versus time. As expected, TD lags behind Ts because the distillate is richer in ethanol. The compositions in the still, xS, and in the distillate, xD, are also plotted. For this transient problem, computations include both the mass and the energy balances. For higher total pressure values, the temperature profiles are shifted to higher values. A constant-heating policy of the still pot is used with q = 2.125 kW.
About
Authors: Housam Binous, Mamdouh Al-Harthi, Brian G. Higgins. Open content licensed under CC BY-NC-SA.
View the source code for this simulation



