Enthalpy and Entropy Departure Functions

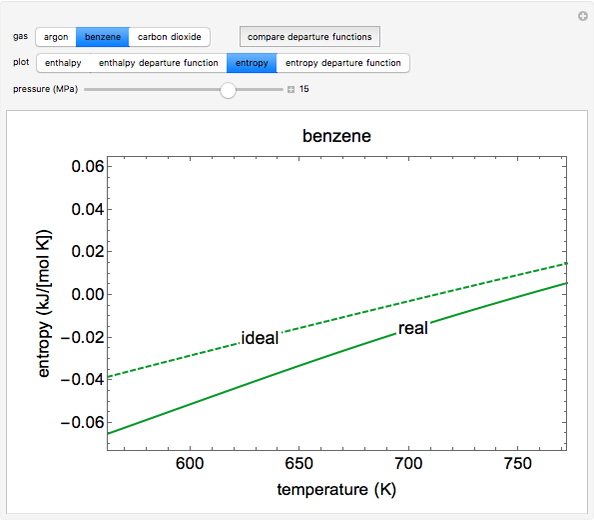
This simulation calculates the enthalpies and entropies for a real gas and an ideal gas as a function of temperature, relative to a reference state at a selected pressure and temperature, using the Peng–Robinson equation of state and ideal-gas heat capacities. The departure function, which is the difference between the thermodynamic property (enthalpy, entropy) for a real gas and an ideal gas at the same temperature and pressure, is also calculated. You can vary the pressure with the slider and select argon, benzene, or carbon dioxide. Select “compare all” to view departure functions for all three gases plotted as a function of temperature at 10 MPa. The departure functions in this plot are shown only above the critical temperature for each gas. Click “show labels” to associate each curve with a molecule.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation



