Chemical Potential Dependence on T and P

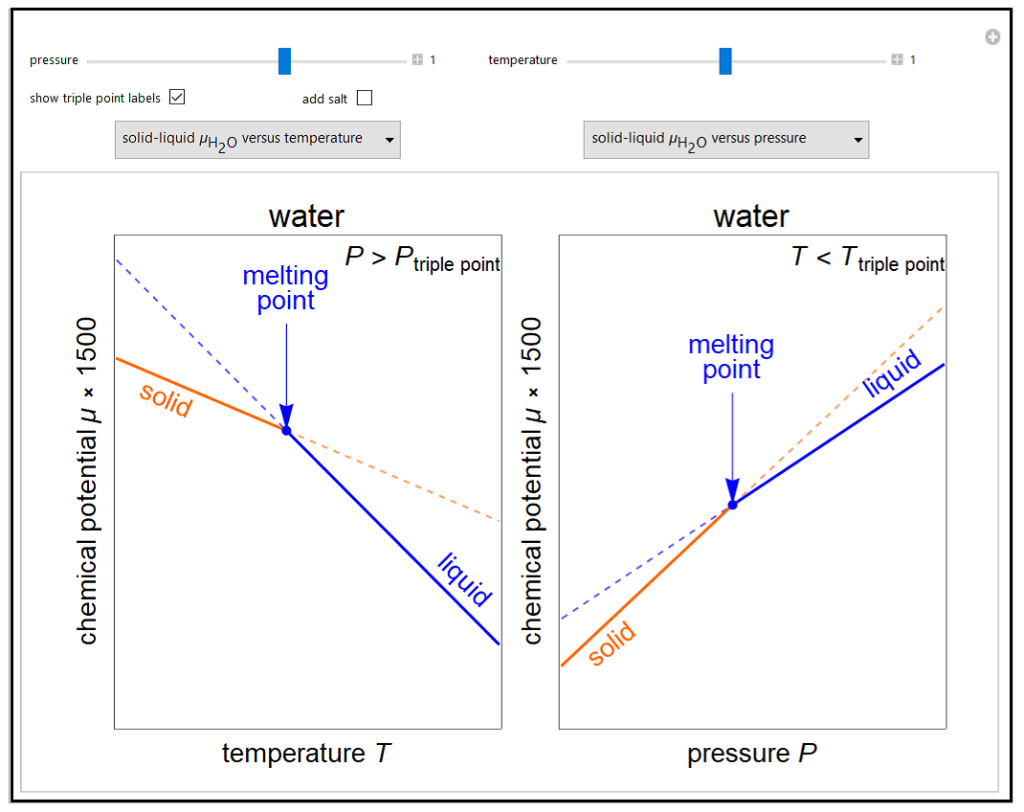
Changes in the chemical potential μ of water as a function of pressure P (at constant temperature T) or temperature (at constant pressure P) determine vapor-liquid, vapor-solid and liquid-solid phase changes. Chemical potential is given by dμ = dG = VdP – SdT. Because the x-axes cover narrow ranges of temperature and pressure, the chemical potential plots are linear. Since the objective of this simulation is to show the qualitative behavior, the temperature or pressure is shown on dimensionless relative scales. Also, note that the y-axis scale is not the same for all plots so that differences in chemical potential are easier to see. Chemical potential as a function of pressure is also shown for the solid-liquid phase change for ethanol, which has a different pressure dependence than water. Use the drop-down menus to select which two plots are displayed. The more stable phases (solid lines) have a lower chemical potential. Use sliders to set temperature for the μ versus P plots or to set pressure for the μ versus T plots. Note that the sliders change the temperature and pressure over narrow ranges. Check “add salt” to see the effect of adding salt to liquid water, and set the salt concentration with a slider. Adding salt leads to boiling point elevation and freezing point depression. The phase changes that occur depend on the pressure or temperature relative to the triple point; check “show triple point labels” to show these on the plots.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Majed N. Aldossary
View the source code for this simulation



