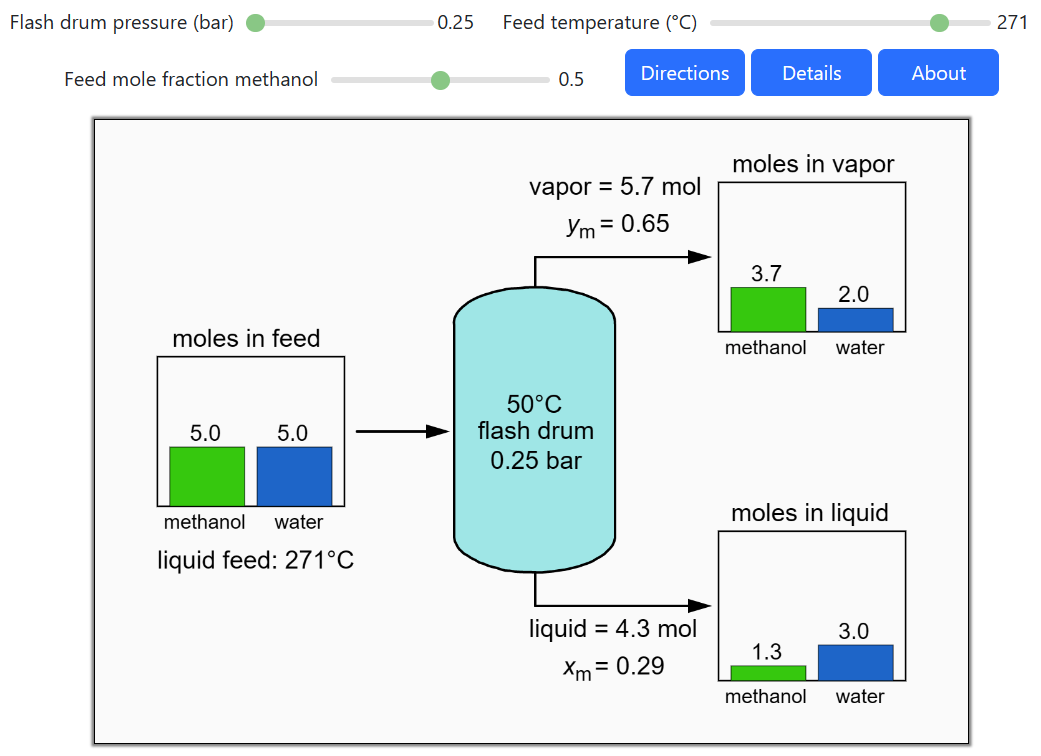
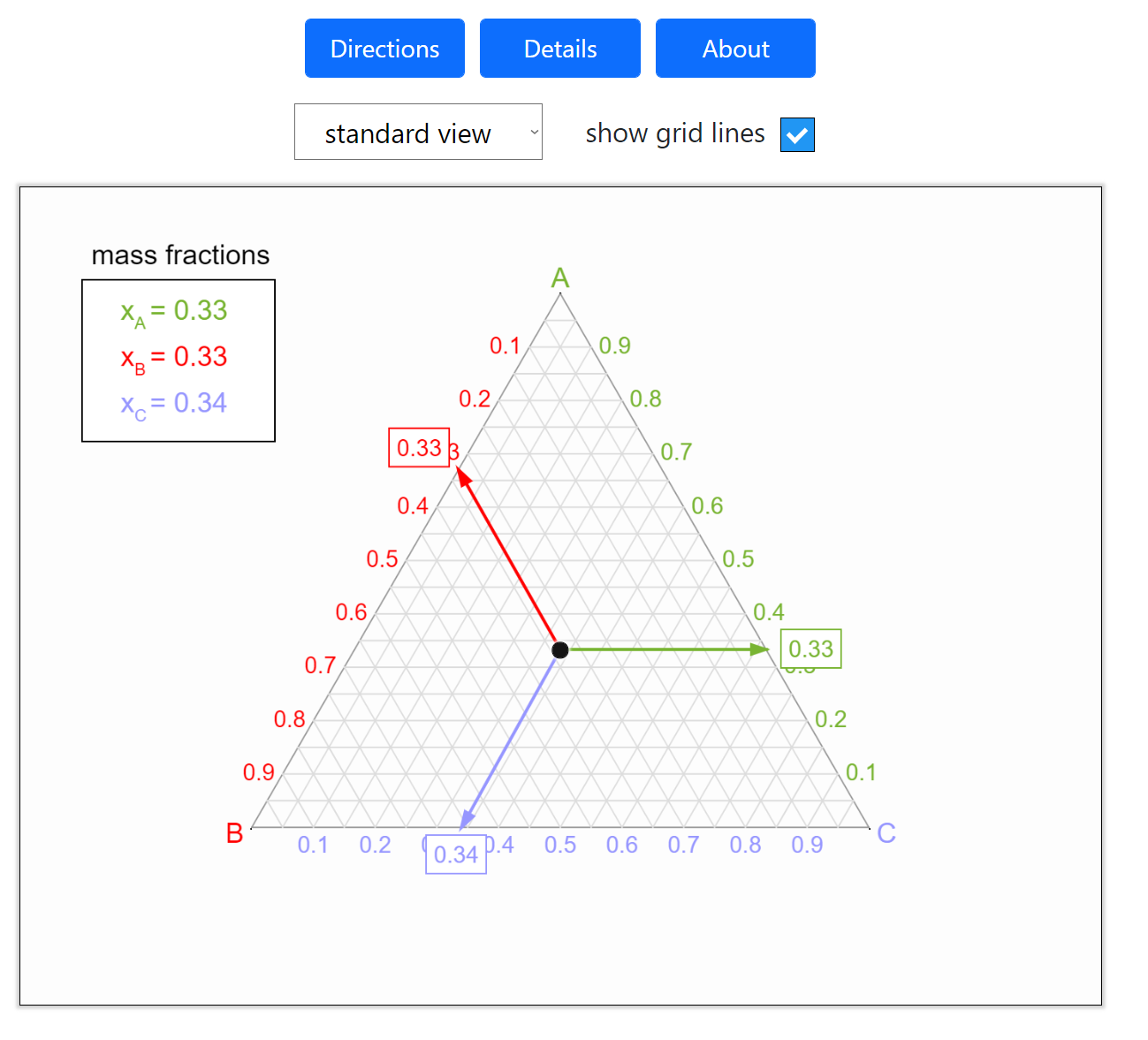
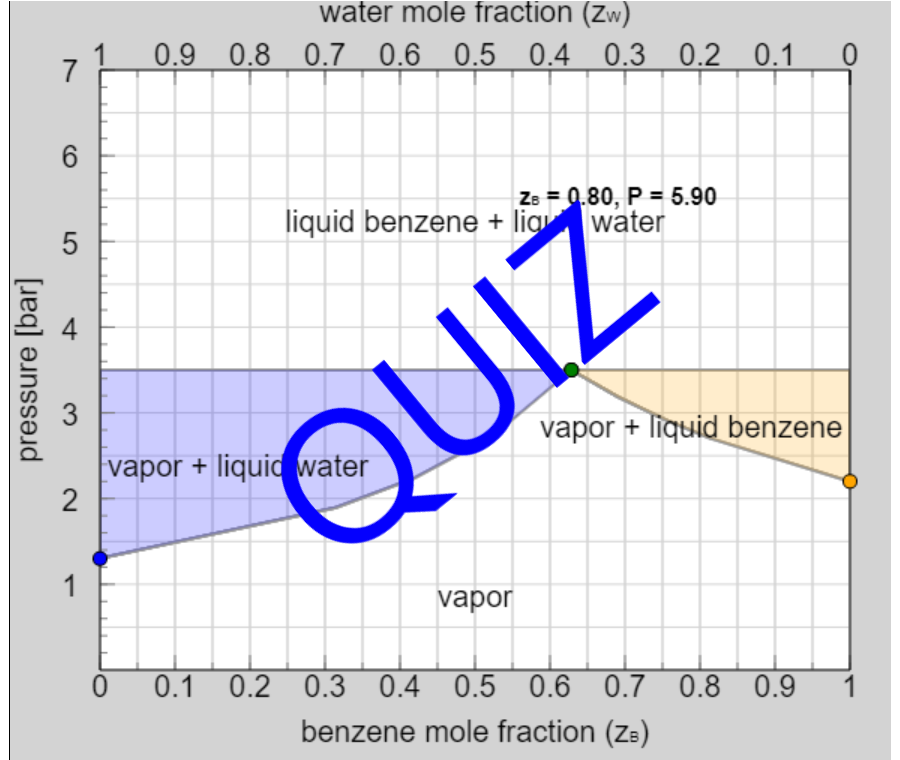
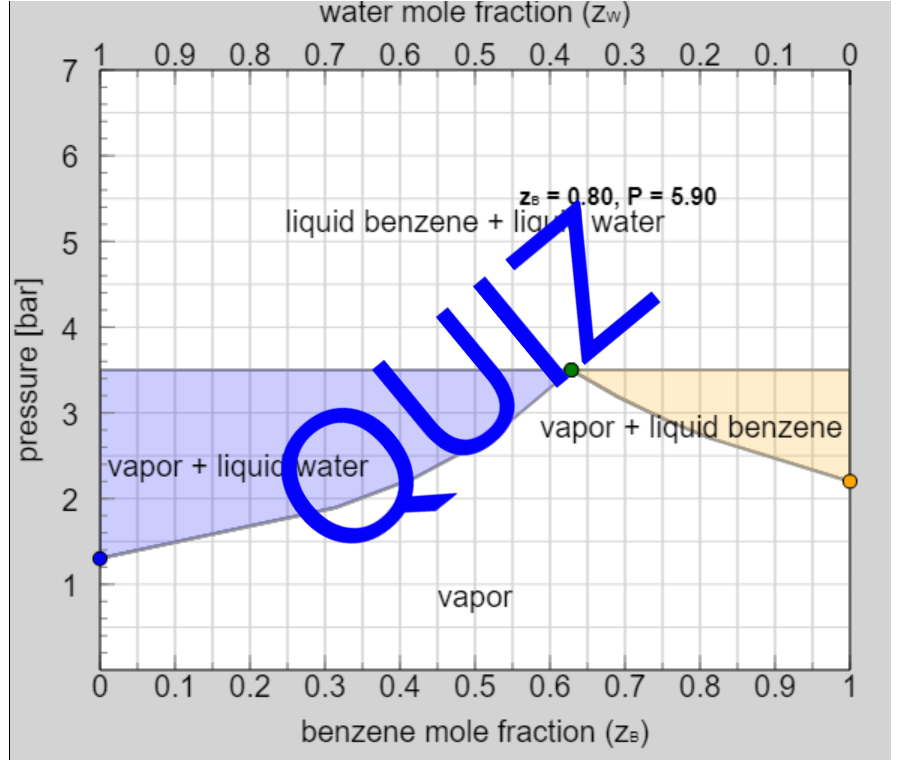
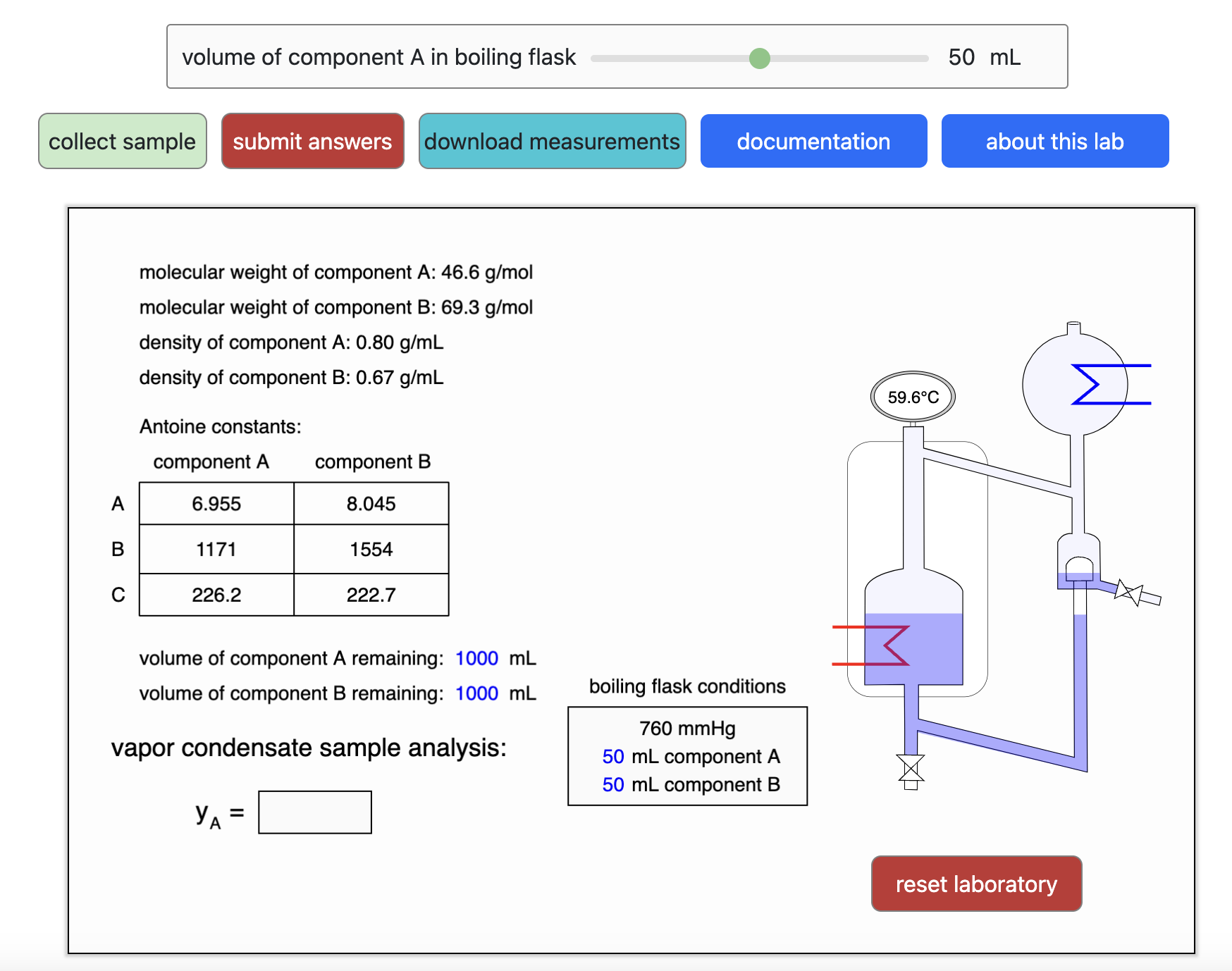
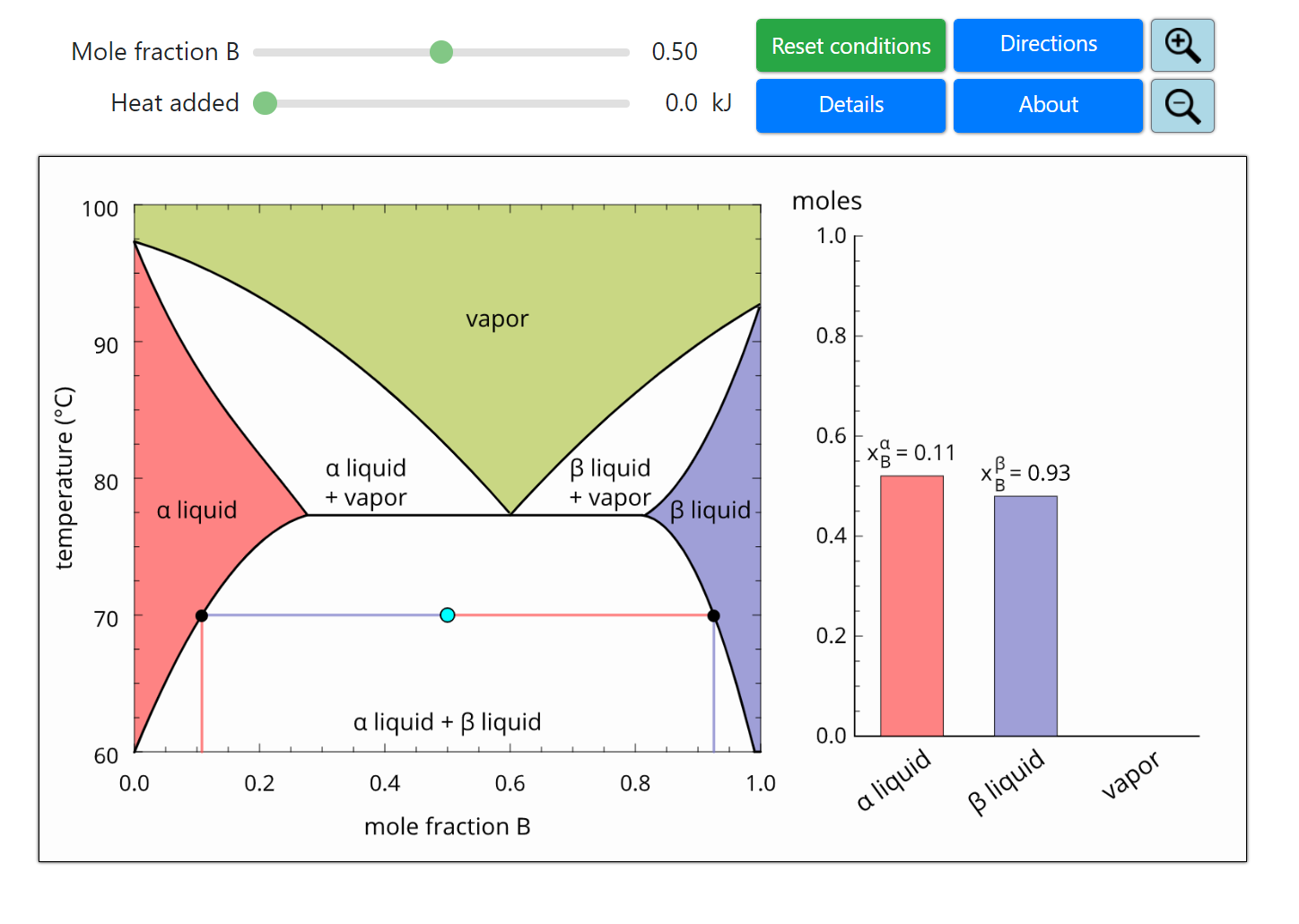
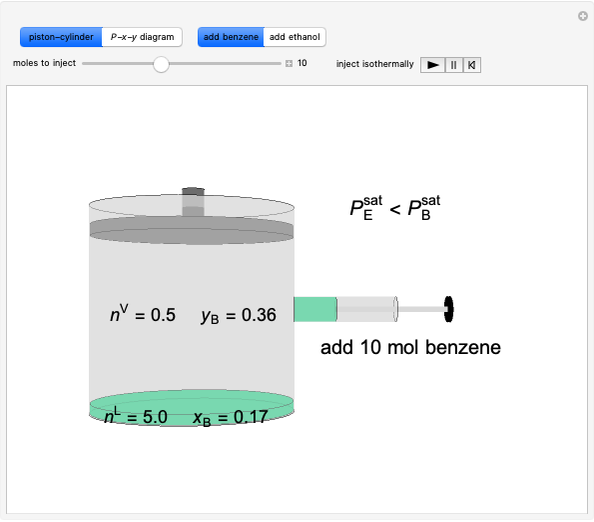
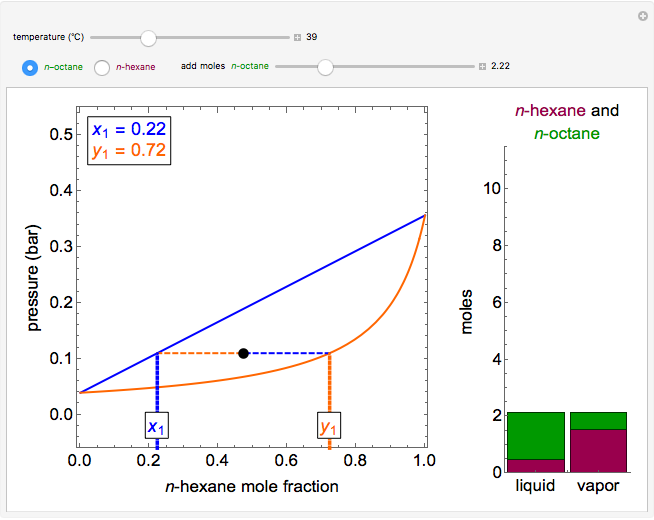
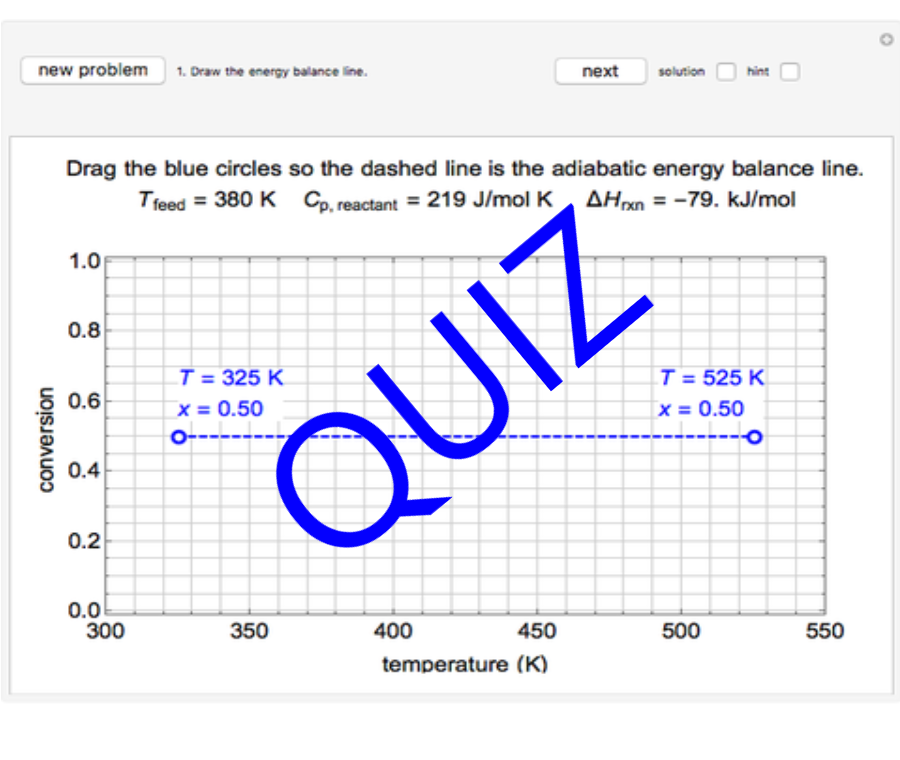
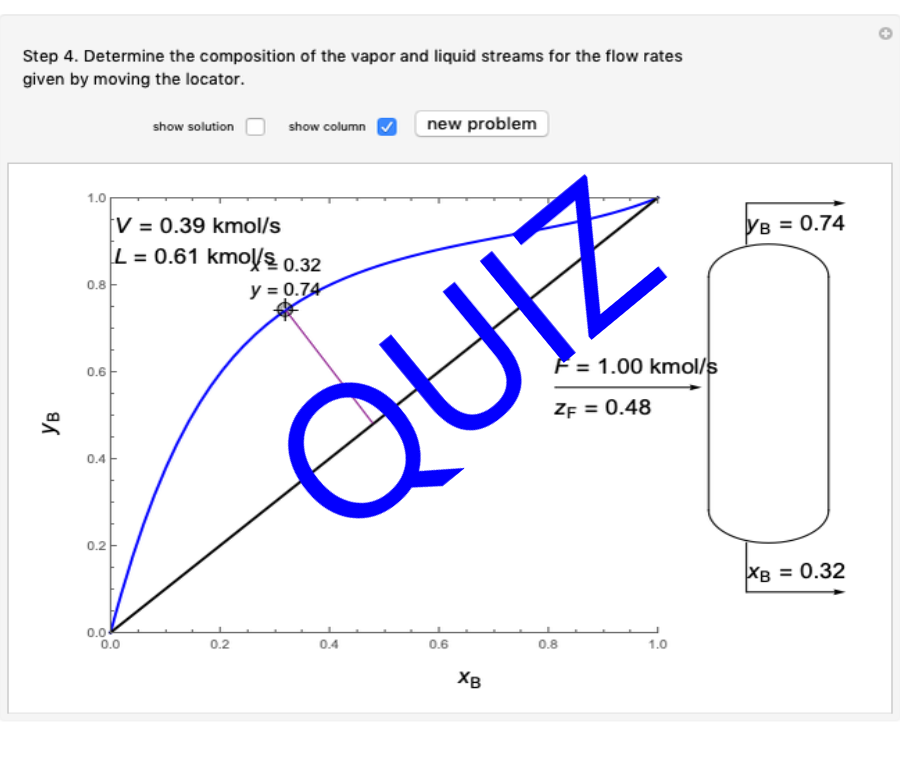
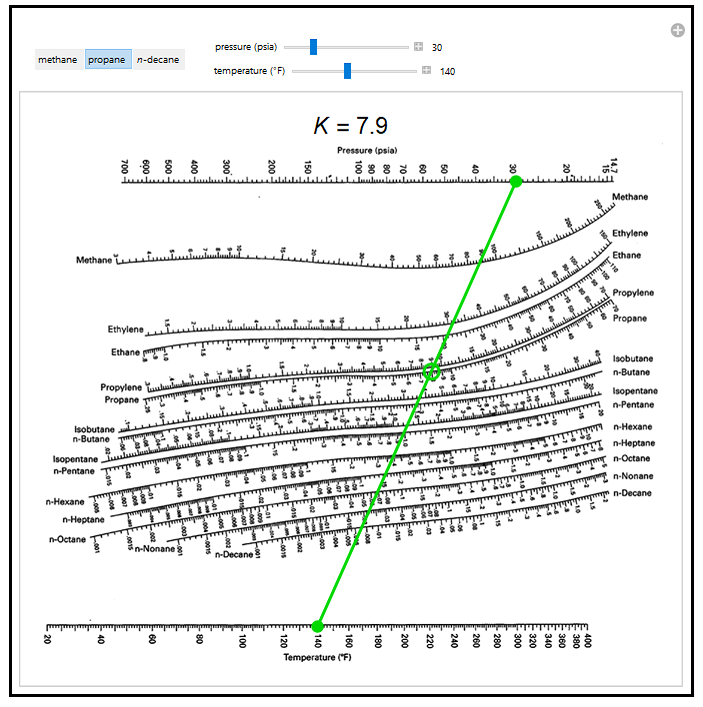
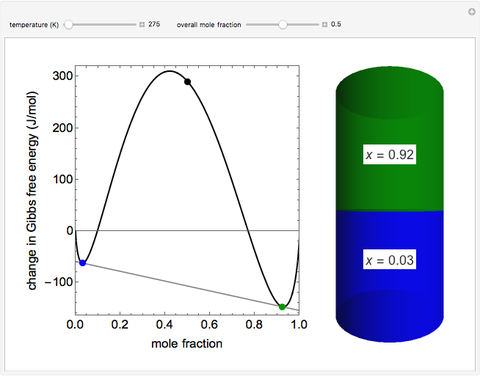
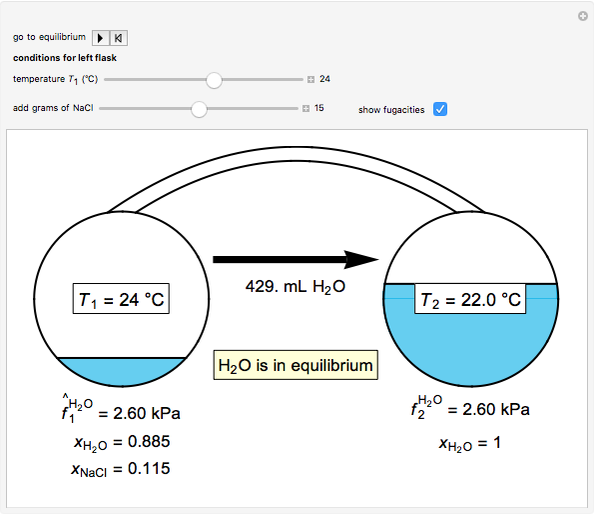
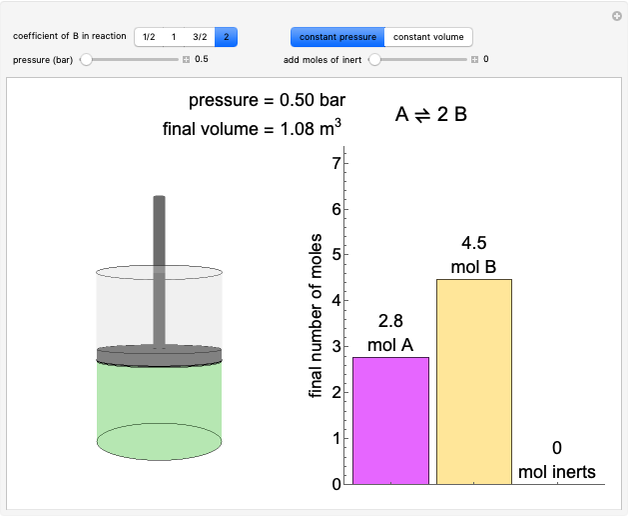
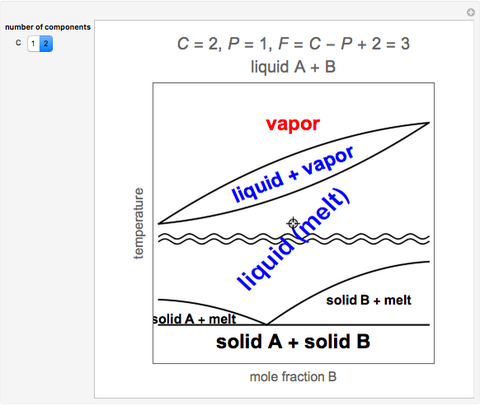
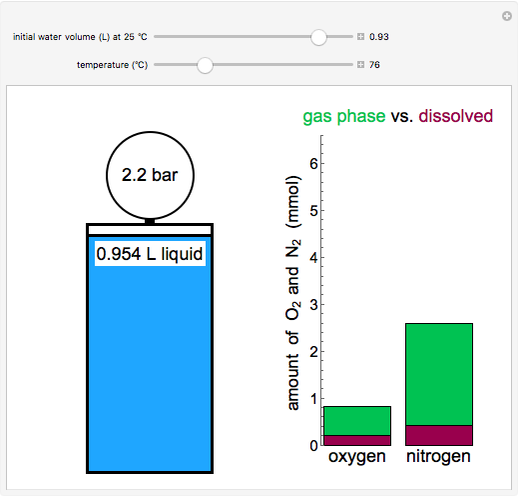
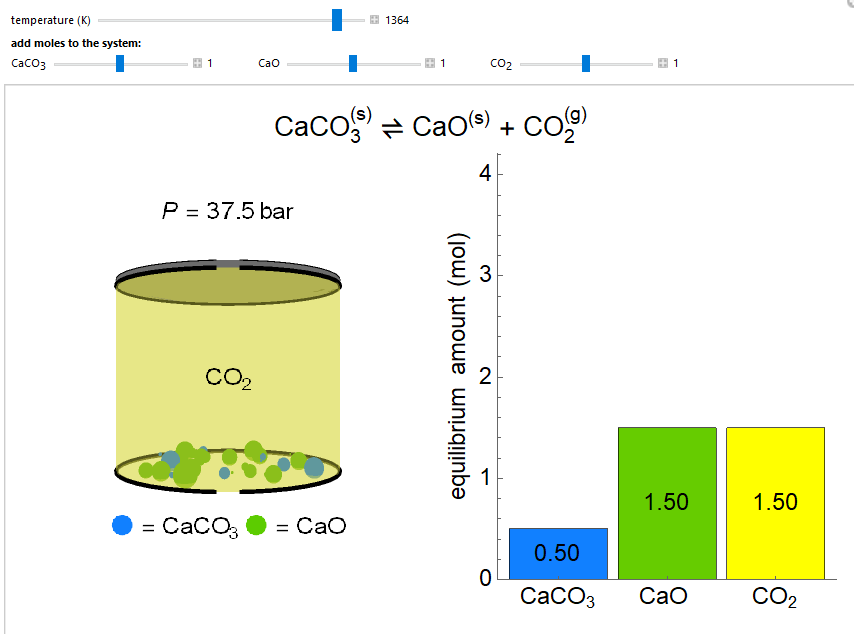
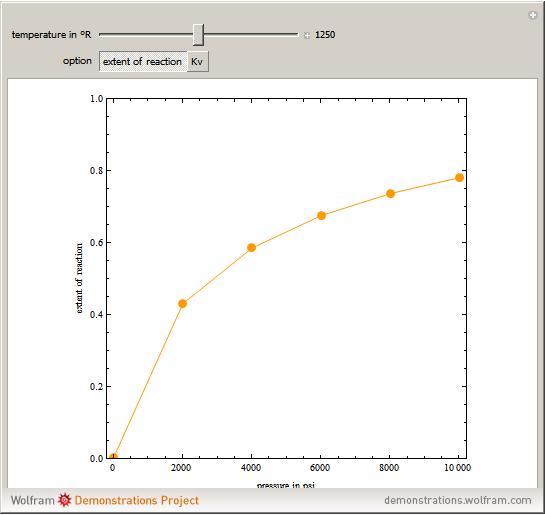
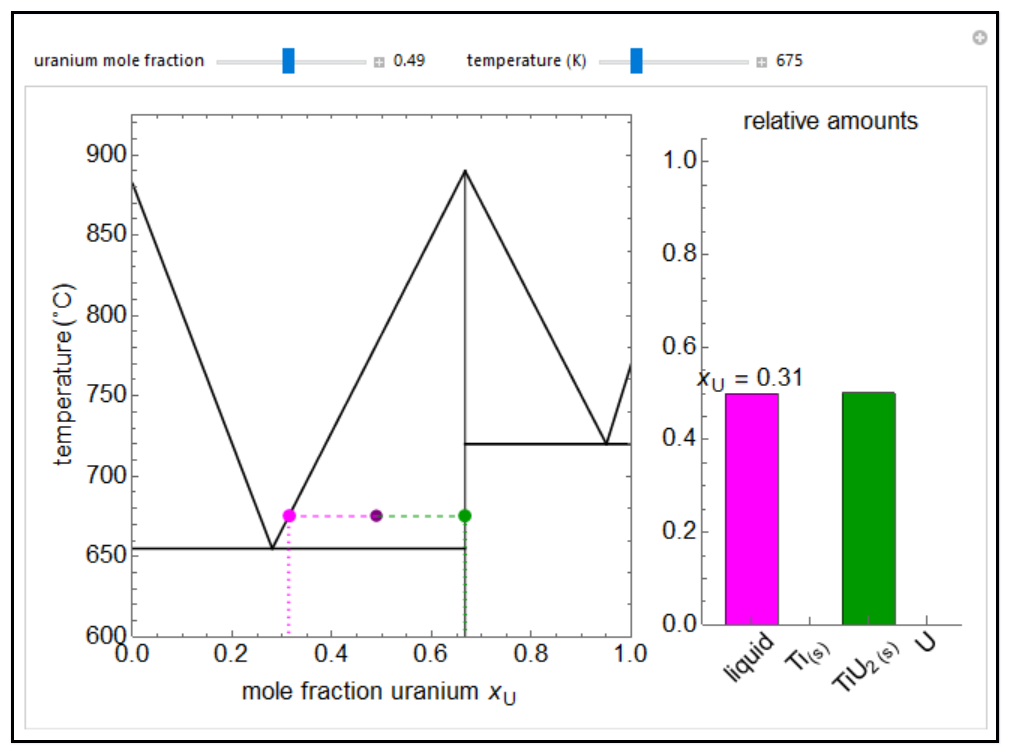
Thermodynamics 2 Simulations
- Select a category
- All simulations
- In-browser simulations
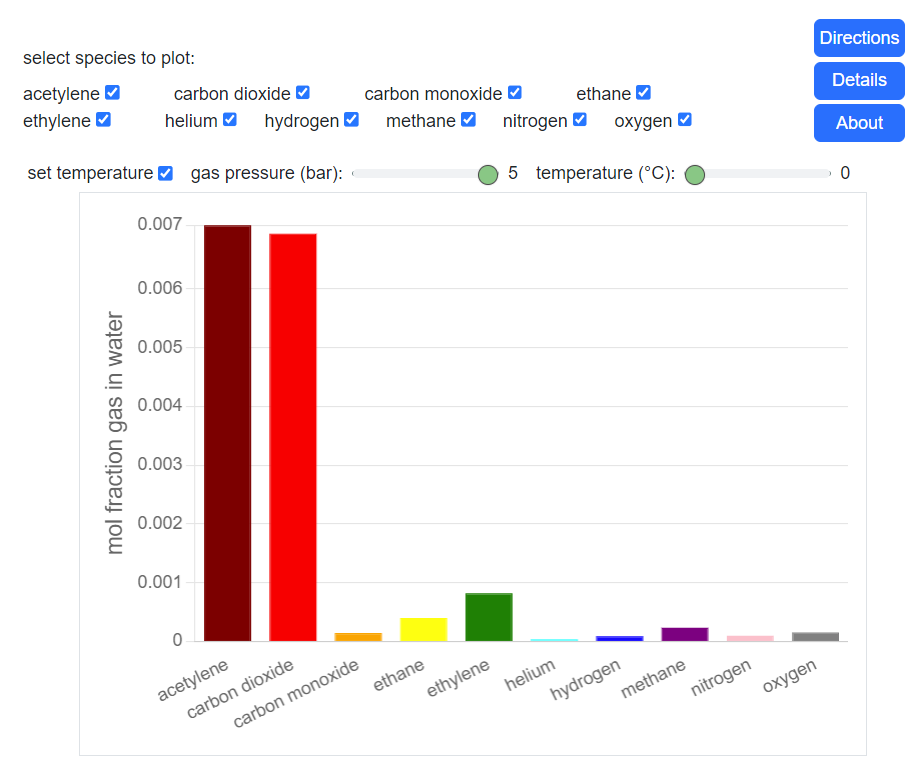
- Fugacity and chemical potential
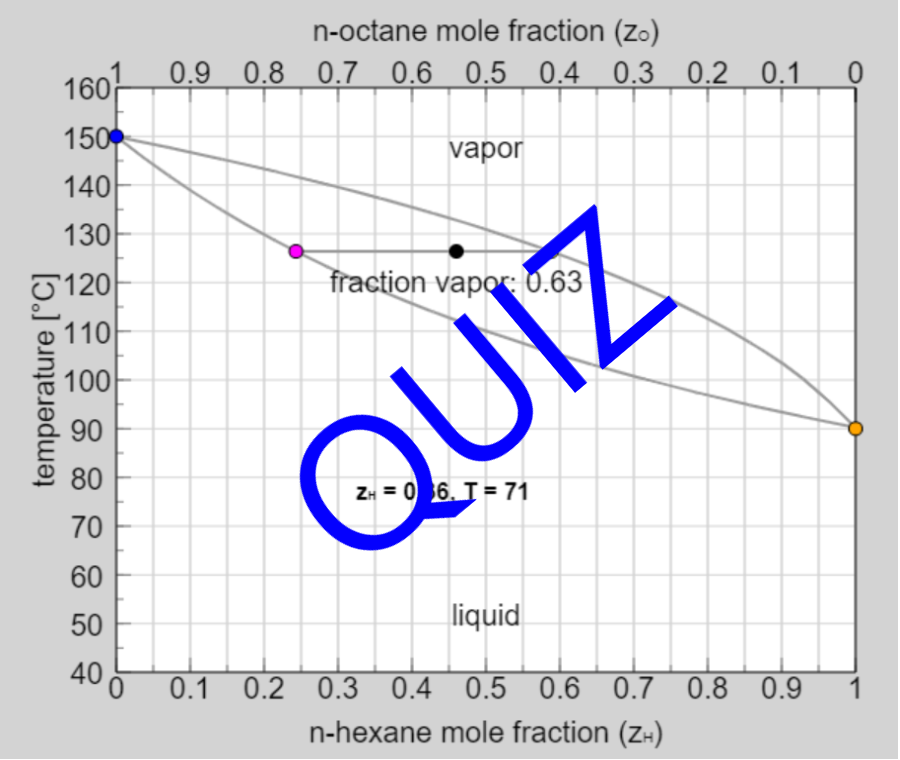
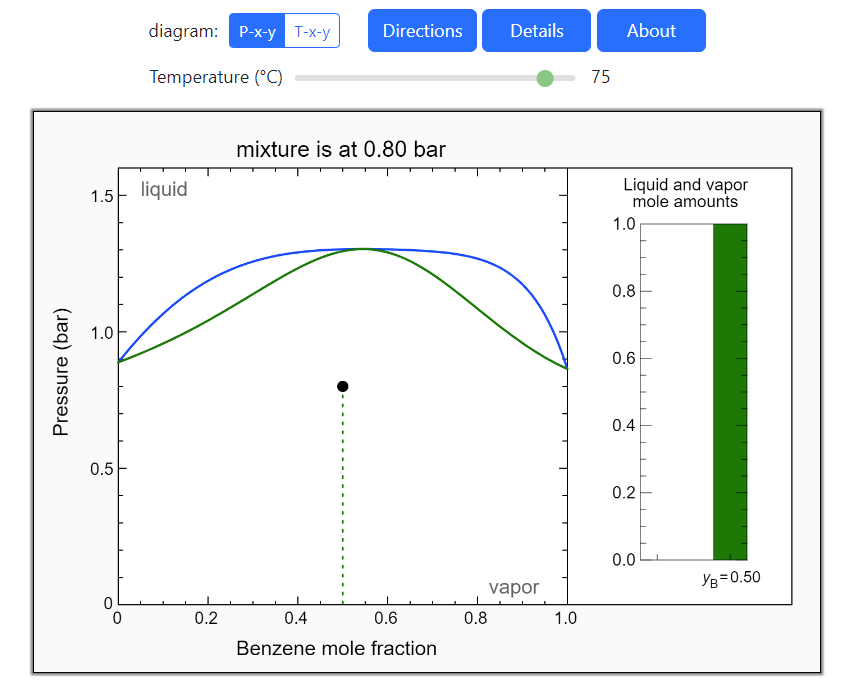
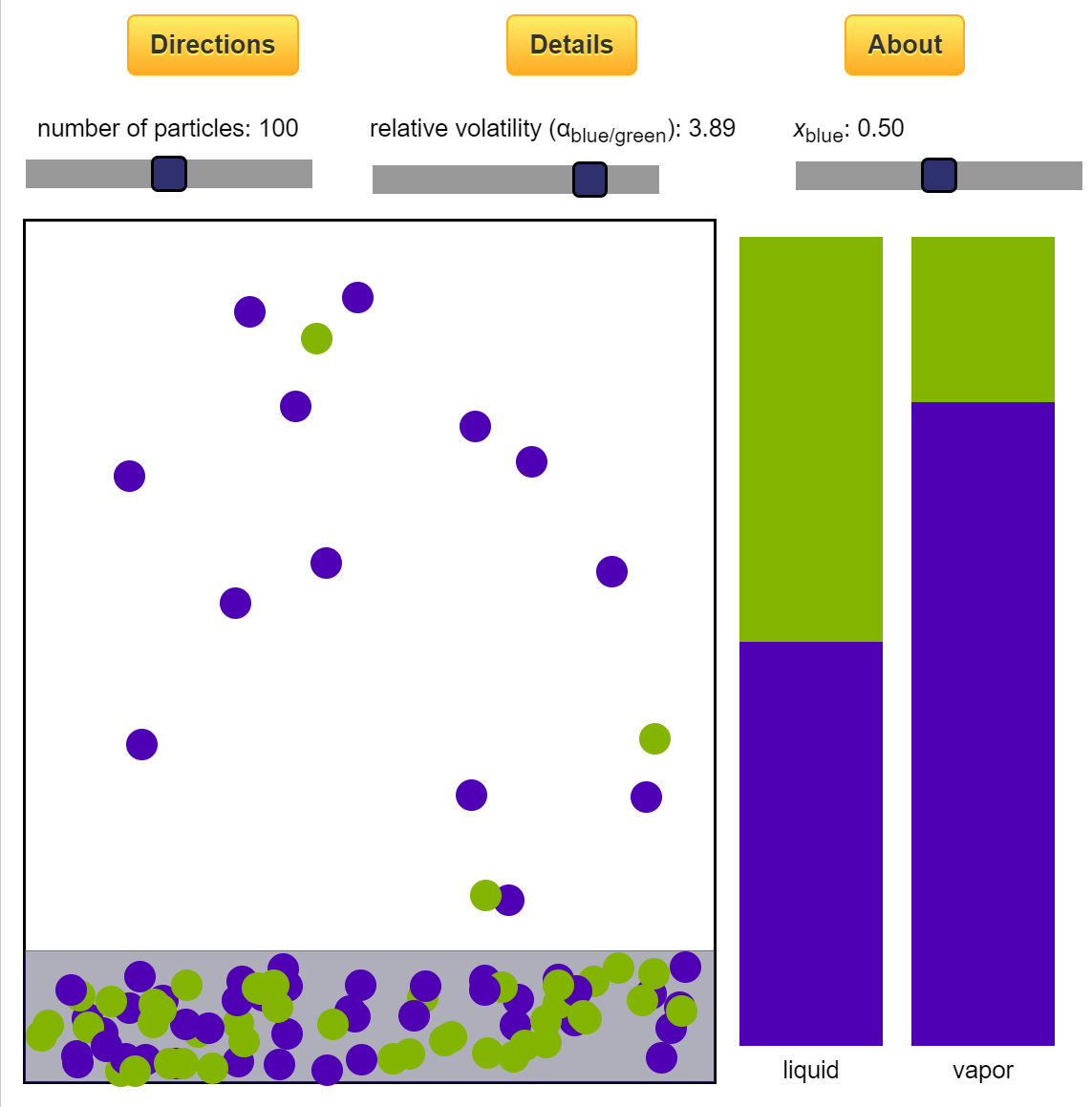
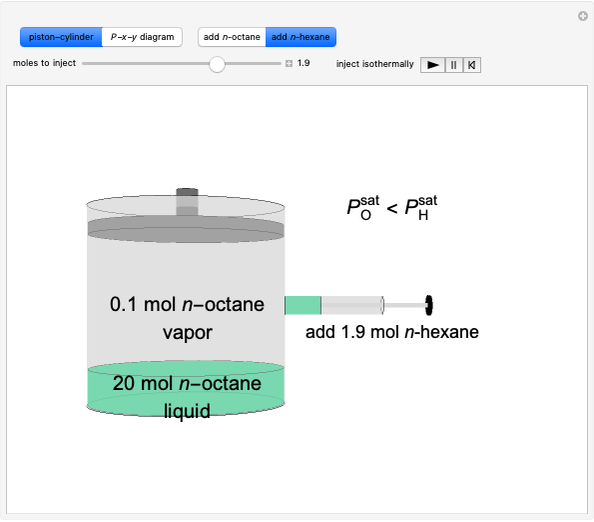
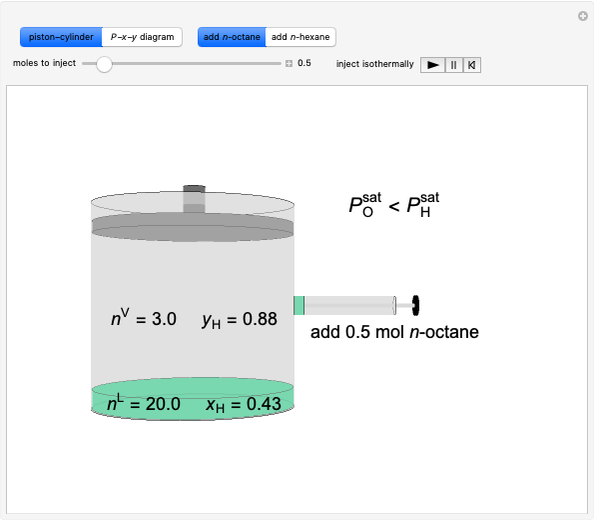
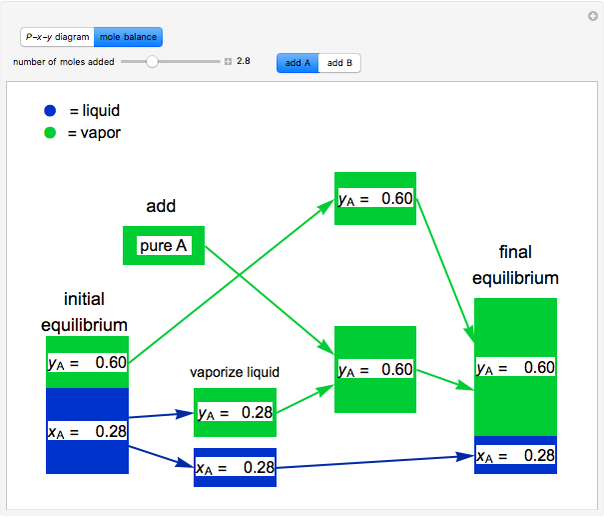
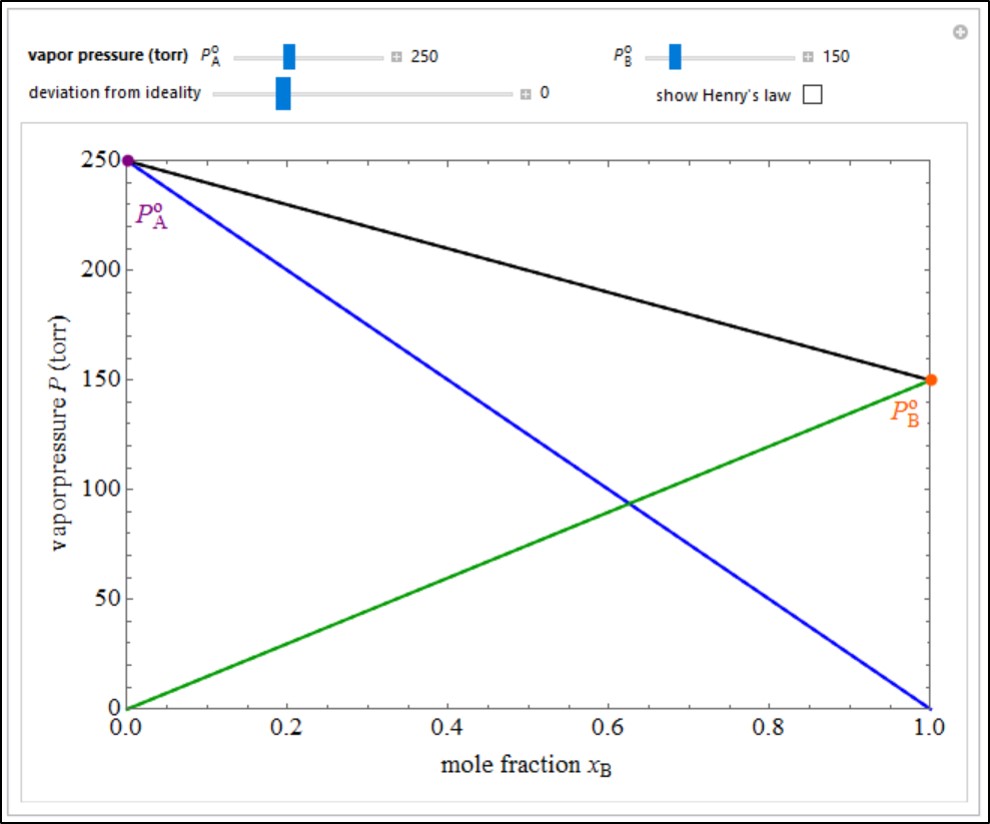
- Multi-component vle for ideal solutions
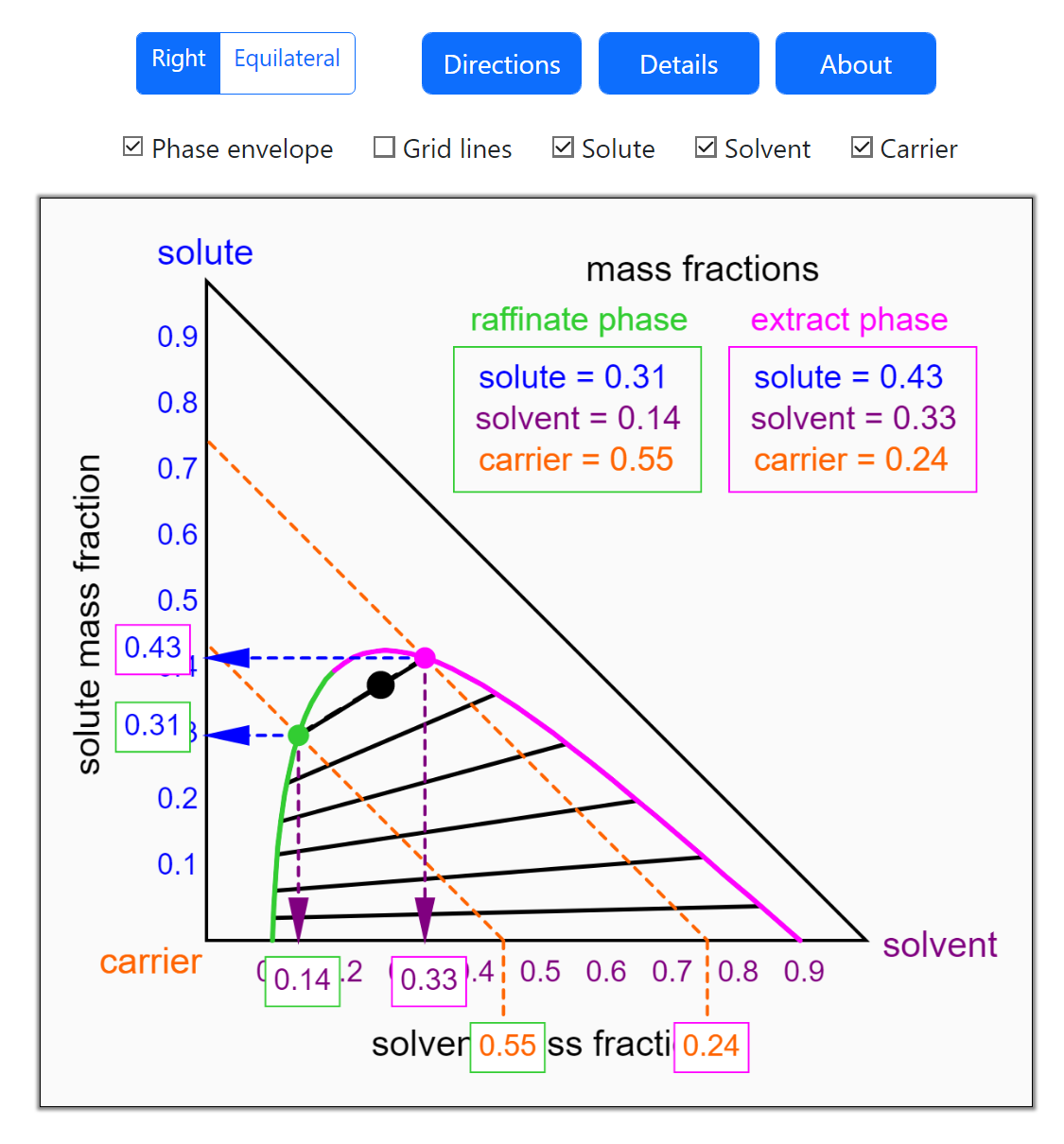
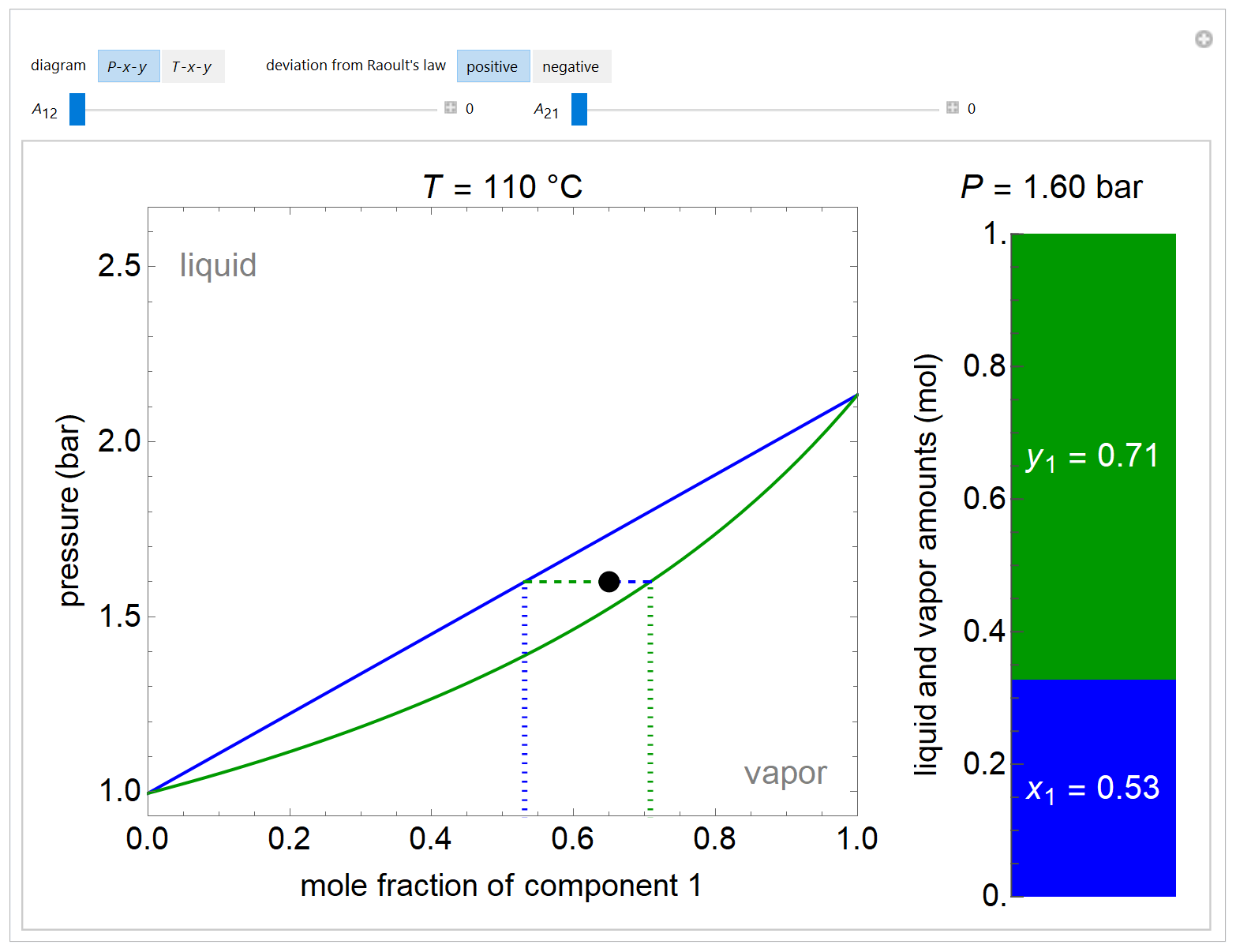
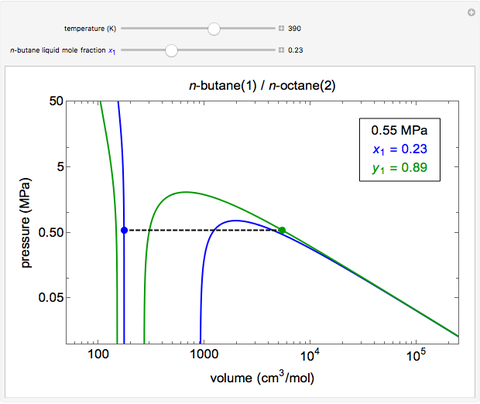
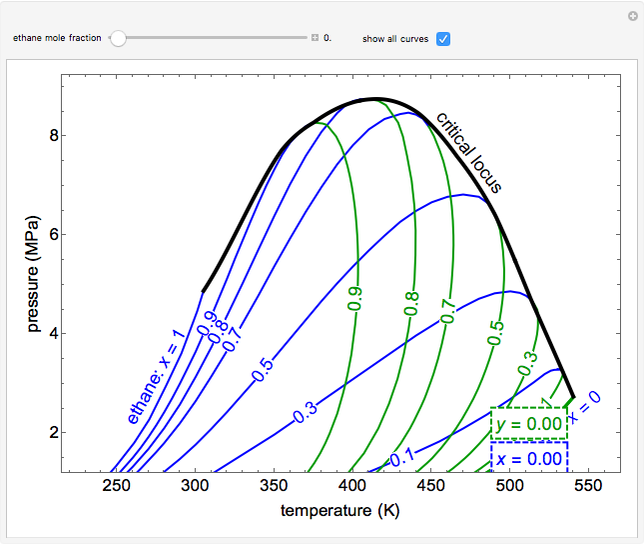
- Multi-component vle for non-ideal solutions
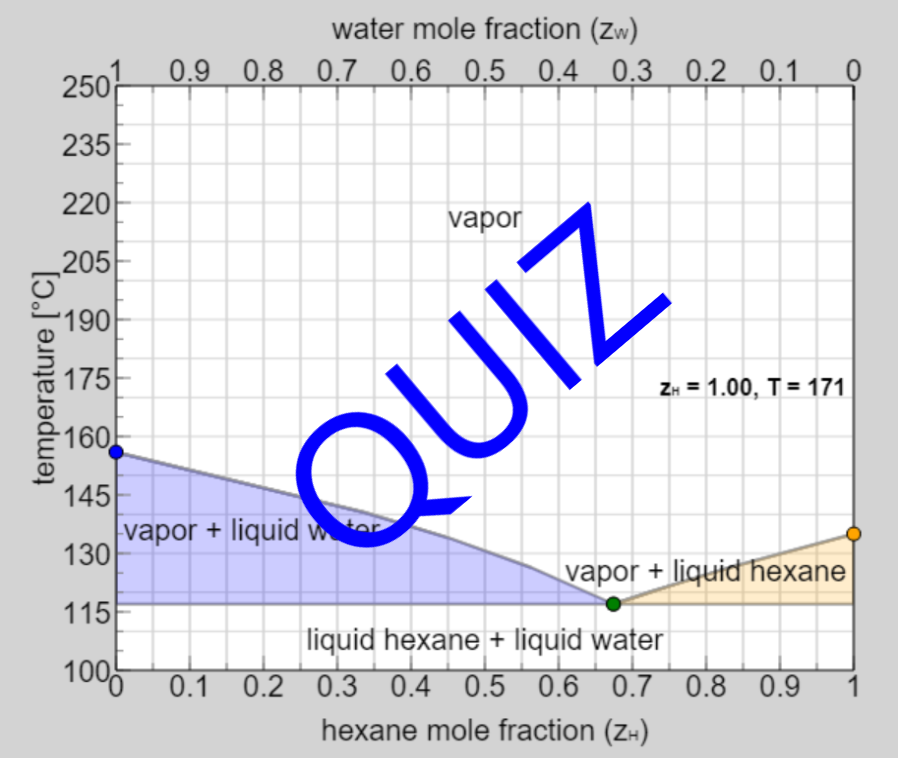
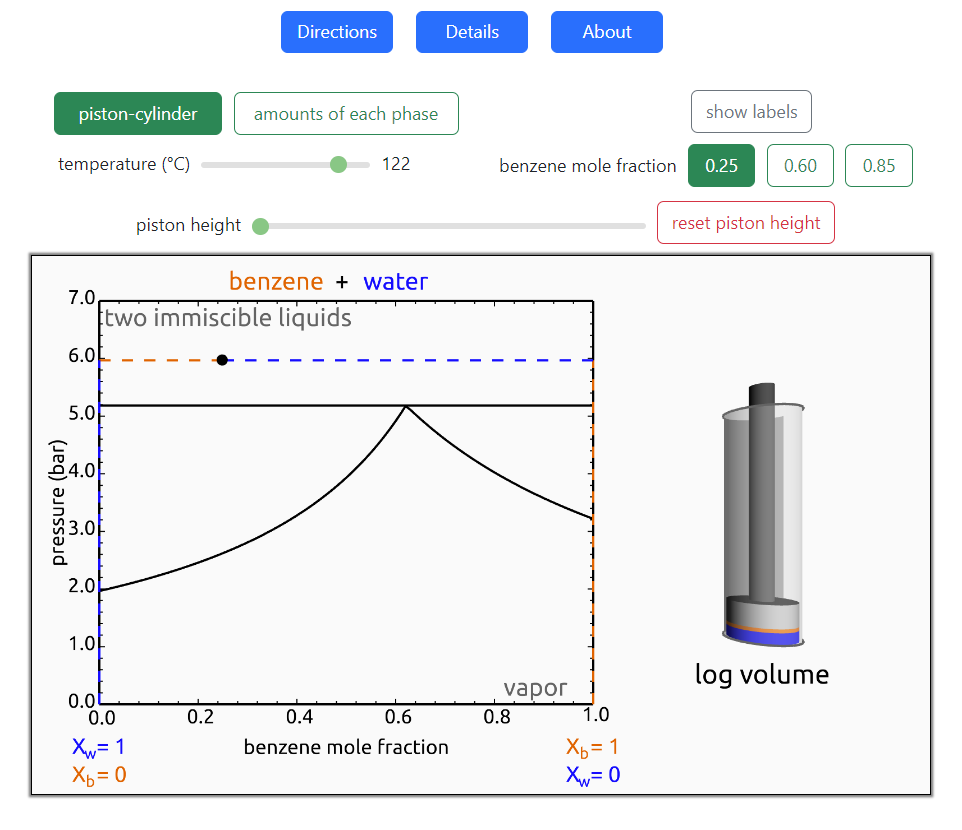
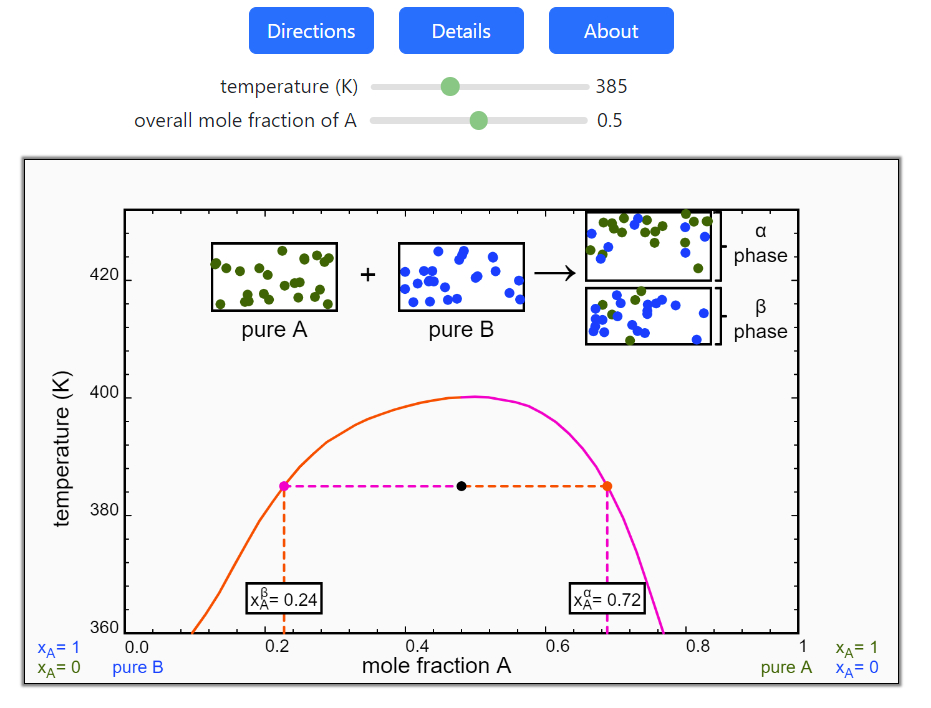
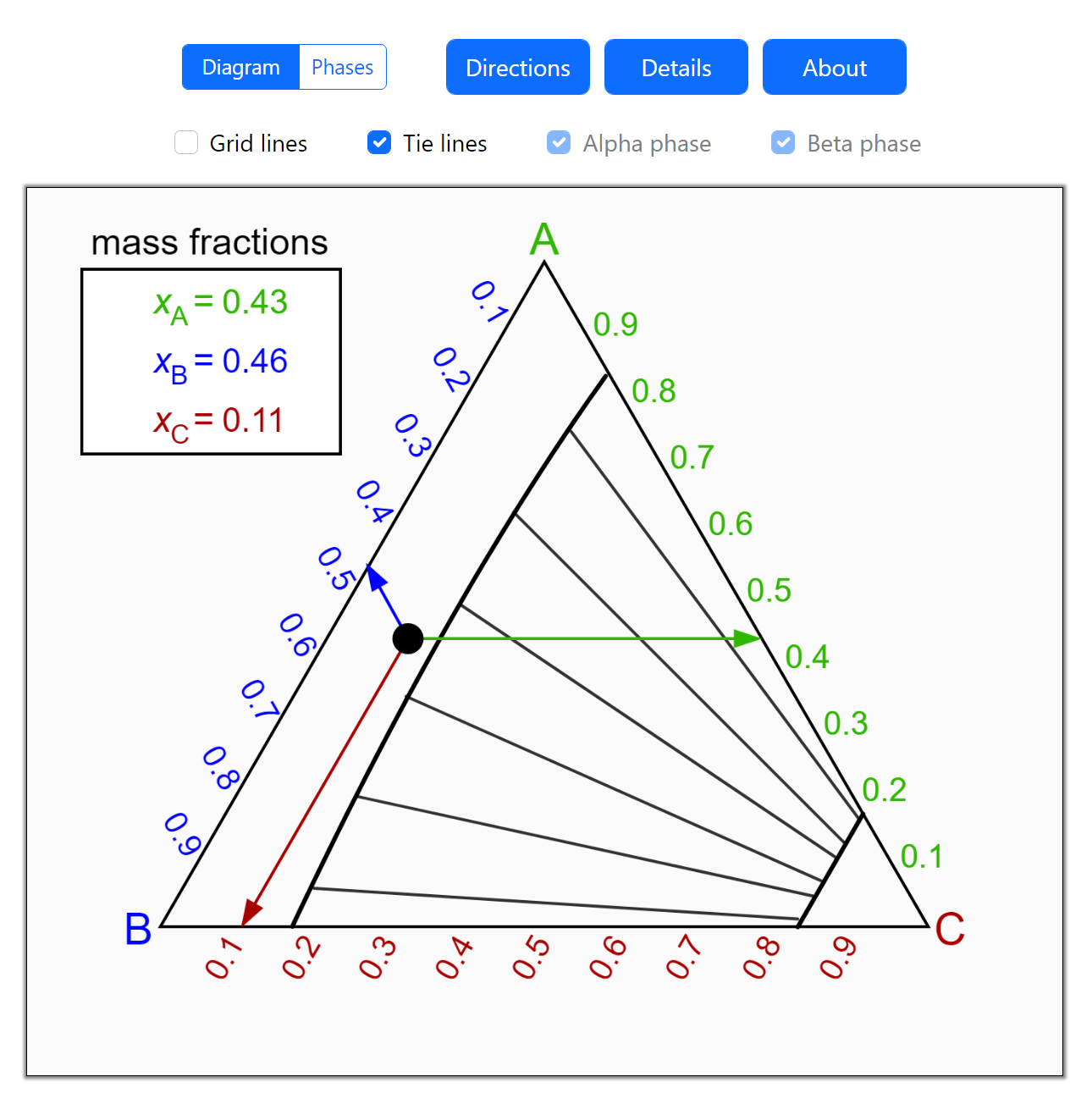
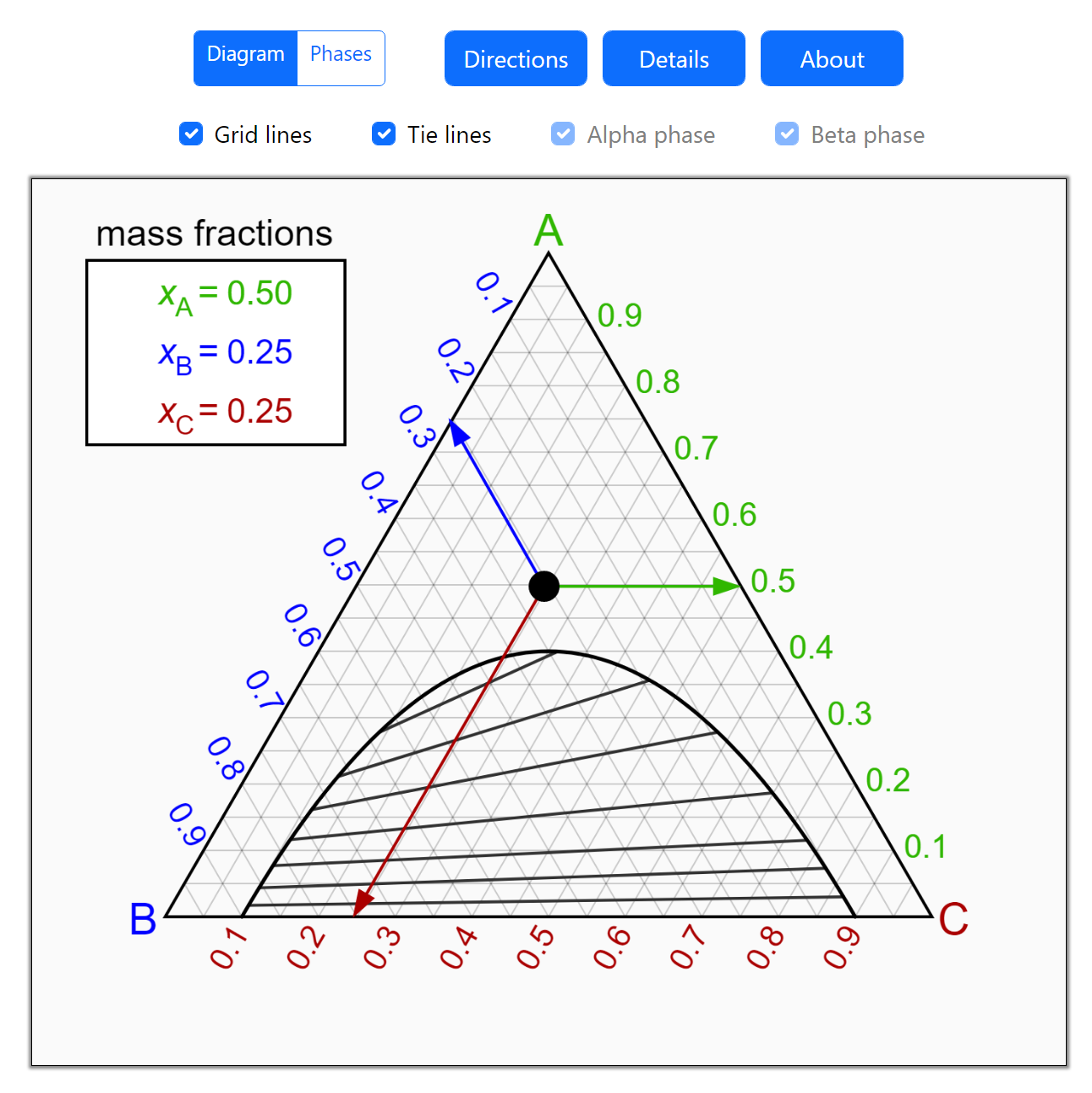
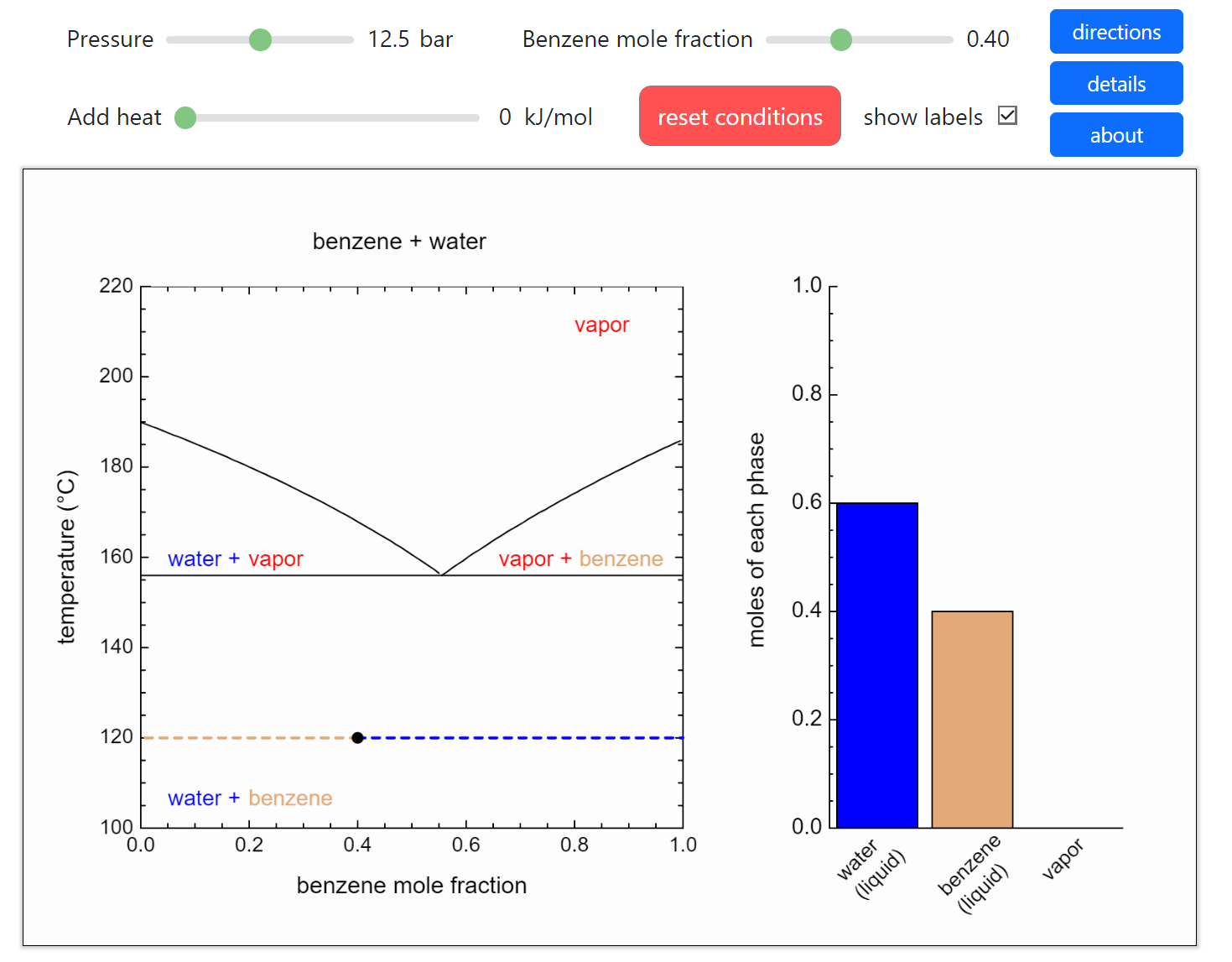
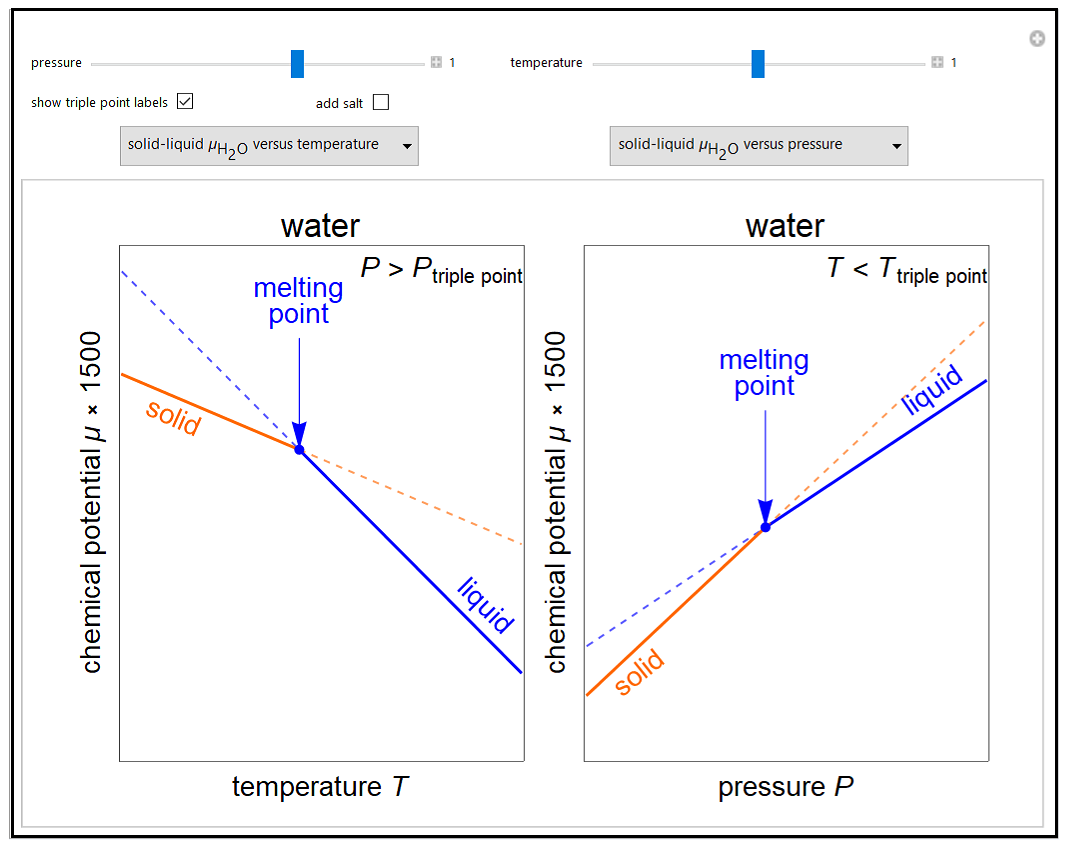
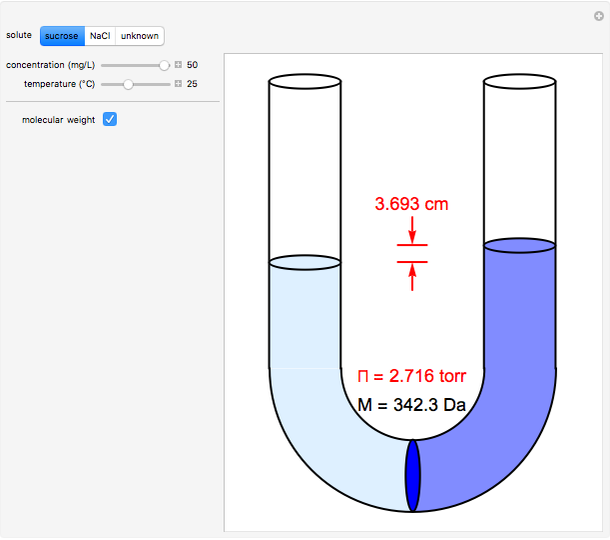
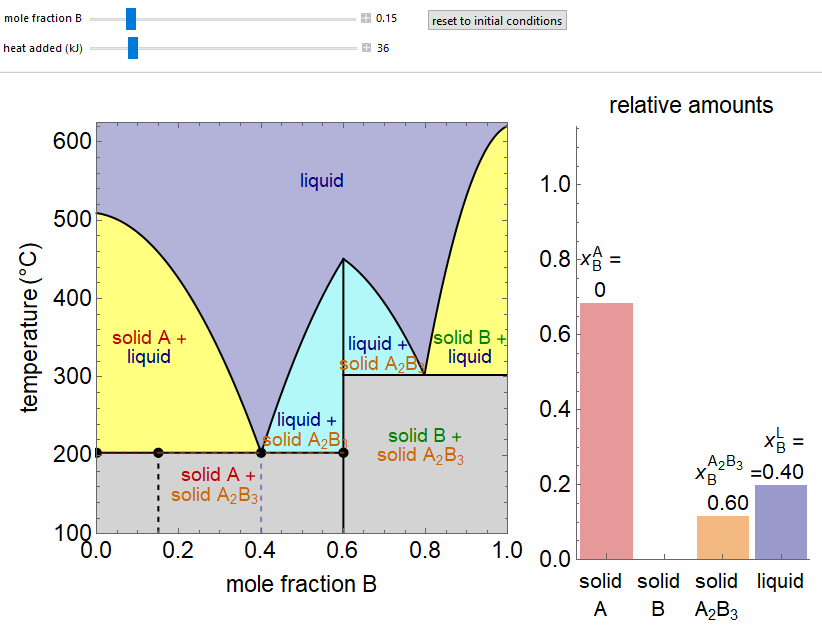
- Partially miscible and immiscible solutions
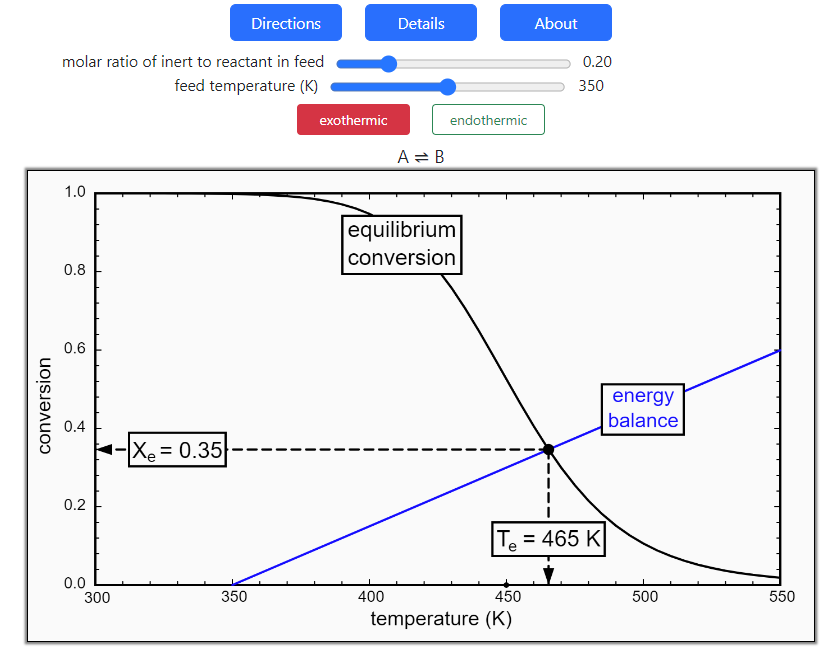
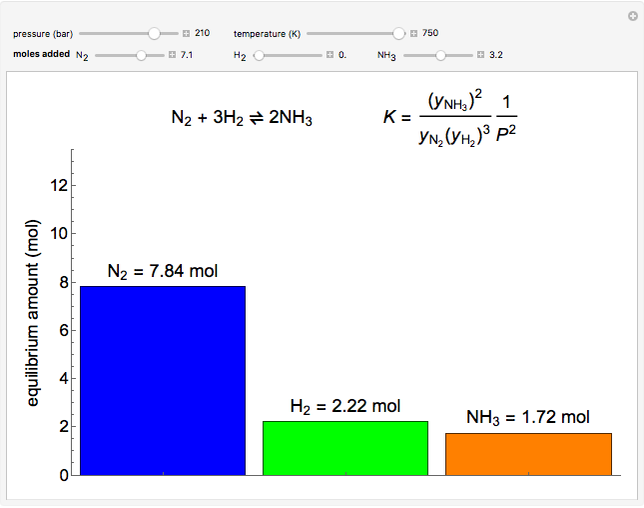
- Reaction equilibrium
No Items Found