Fugacities in a Can of Soda

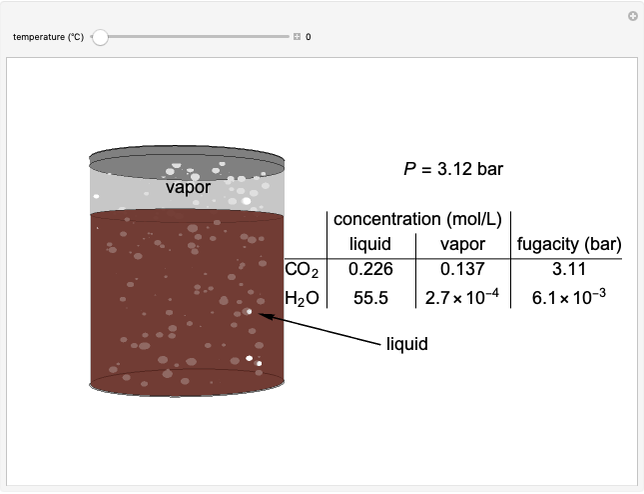
The fugacities of water and carbon dioxide are calculated as a function of temperature for a closed container, which is a model of a can of soda. The concentrations of the two components are calculated in both the liquid and the gas phases. As the temperature increases, the pressure increases, and therefore the fugacities increase. Note that the CO2 concentration is much lower than the H2O concentration in the liquid phase, but the CO2 concentration is much higher than the H2O concentration in the gas phase. Because the gas phase is assumed to be ideal, the fugacities of CO2 and H2O in both phases are equal to their gas-phase partial pressures, and thus the CO2 fugacity is much higher than the H2O fugacity. As the temperature increases, the CO2 concentration in liquid water decreases. However, as the temperature increases, the pressure increases, and a higher CO2 pressure increases the CO2 concentration in water, so the net effect is that the concentration in the liquid phase does not change much as the temperature increases.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation



