Peng-Robinson EOS for Mixtures

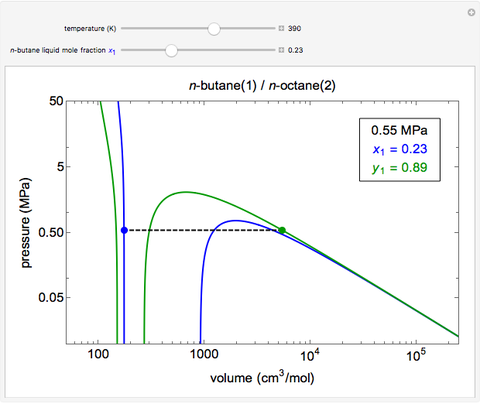
This simulation uses the Peng-Robinson equation of state for mixtures to plot isotherms for n-butane(1)/n-octane(2) mixtures on a log pressure versus log volume graph. Use the sliders to select the temperature. Selecting the mole fraction x1 of the liquid yields the isotherm for the liquid in blue. The green isotherm is for the vapor phase (mole fraction y1) that is in equilibrium with the liquid; the values of y1 and the pressure are determined from Raoult’s law and are displayed in the upper-right corner. The dashed black line connects the blue isotherm to the green isotherm at the VLE pressure.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
