Combustion Reactions: Example Problems
Try to solve these problems before watching the solutions in the screencasts.
Example Problem 1
\[CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O\]
\[CH_4 + \frac{3}{2} O_2 \rightarrow CO + 2H_2O\]
Given:
- Basis of 100 moles methane
- 25% excess air
- Complete conversion of methane
- No partial combustion
Find:
- Moles of air fed to reactor
- Moles of each component in stack gas
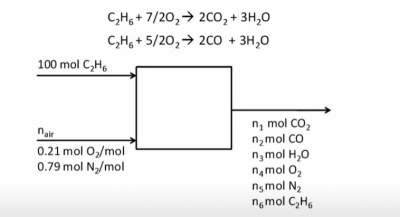
Example Problem 2
Given:
- Basis of 100 moles ethane
- 50% excess air
Find:
- Ratio of water/dry gas in stack gas