Thermodynamics 1 screencasts
Screencasts are organized by textbook on the right.
Energy and entropy
What is thermodynamics?
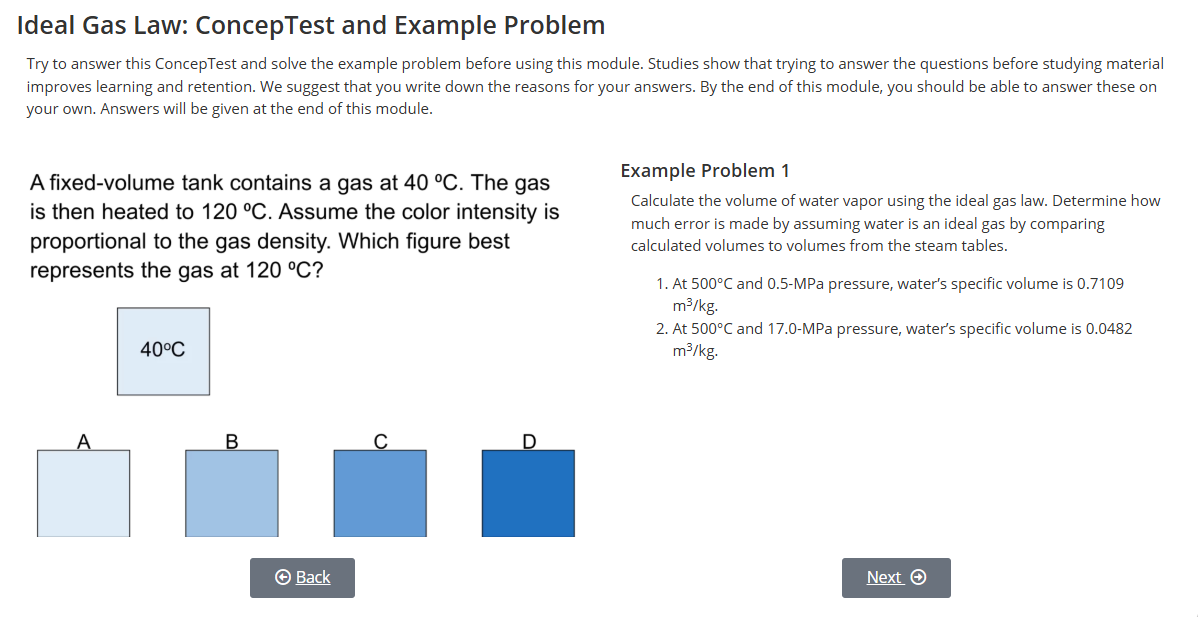
Steam tables
Closed system energy balances
Adiabatic reversible and irreversible processes
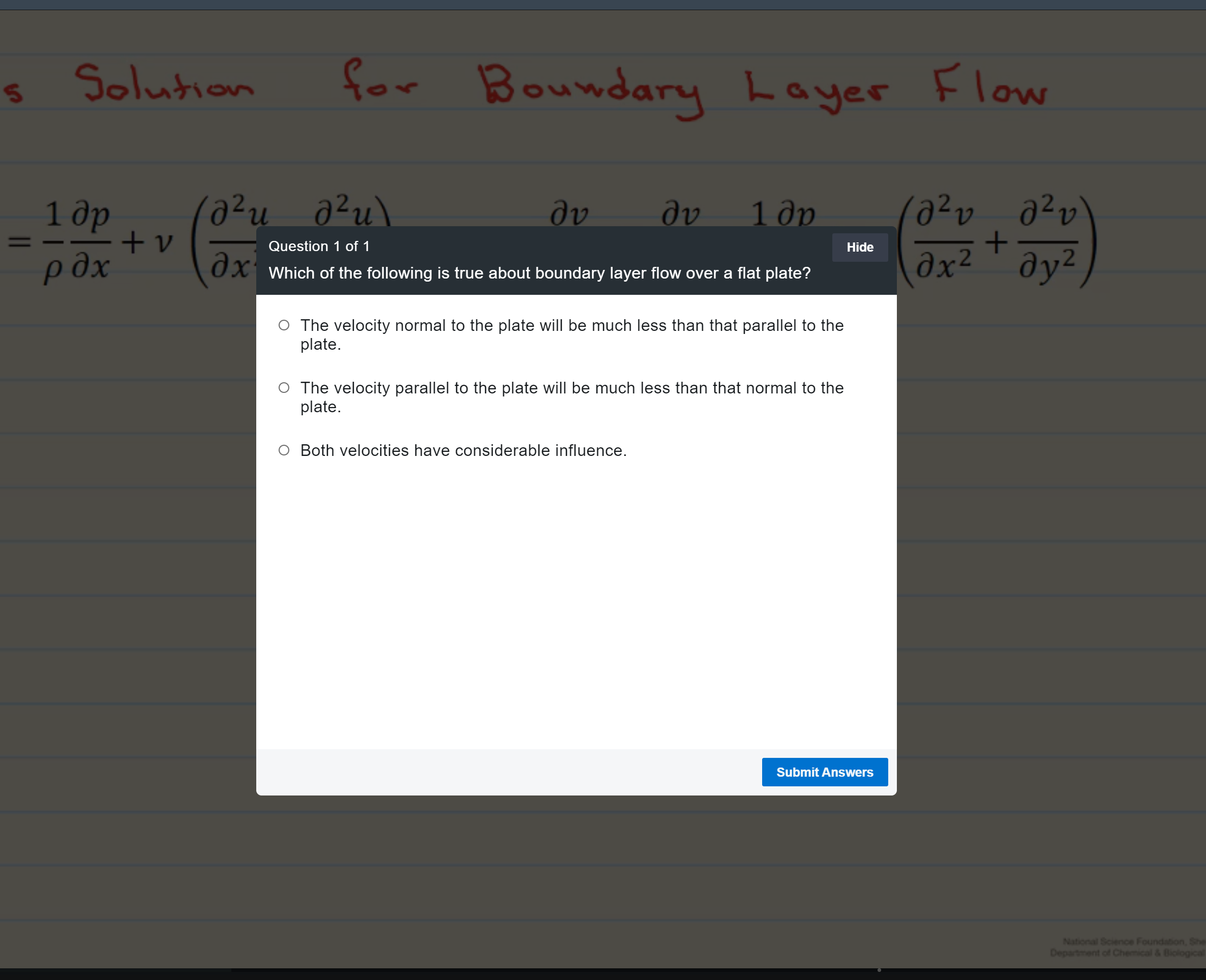
Steady-state, open systems, energy balances
Steady-state, open systems, energy balances-turbines and compressors
Steady-state, open systems, energy balances-throttle/Joule-Thomson expansion
Steady-state, open systems, energy balances-other
Energy balances on unsteady-state open systems
Entropy introduction
Calculate entropy changes
Entropy changes and diagrams
Cycles
Single-component fluid properties
Departure function
Derivatives/differentials/maxwell relations
Equations of state (EOS)
Equations of state (EOS) spreadsheet
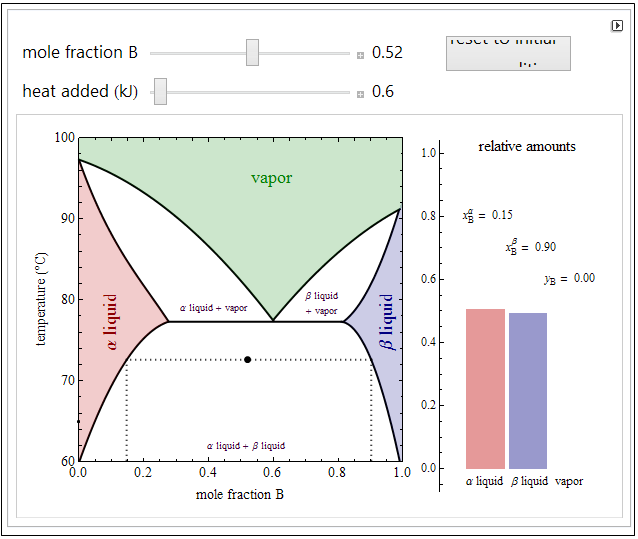
Single-component PVT properties
Single-component phase diagrams
Gibbs free energy/fugacity (single-component)
Chemical potential